News & Events
News

Are you interested in the latest news from D-BIOL, e.g. new papers from department members, events, new faculty and alike? Here you will find the latest news items.
Inaugural and farewell lectures

Lectures held by new faculty members and by those leaving the D-BIOL are listed here.
D-BIOL seminar calendar
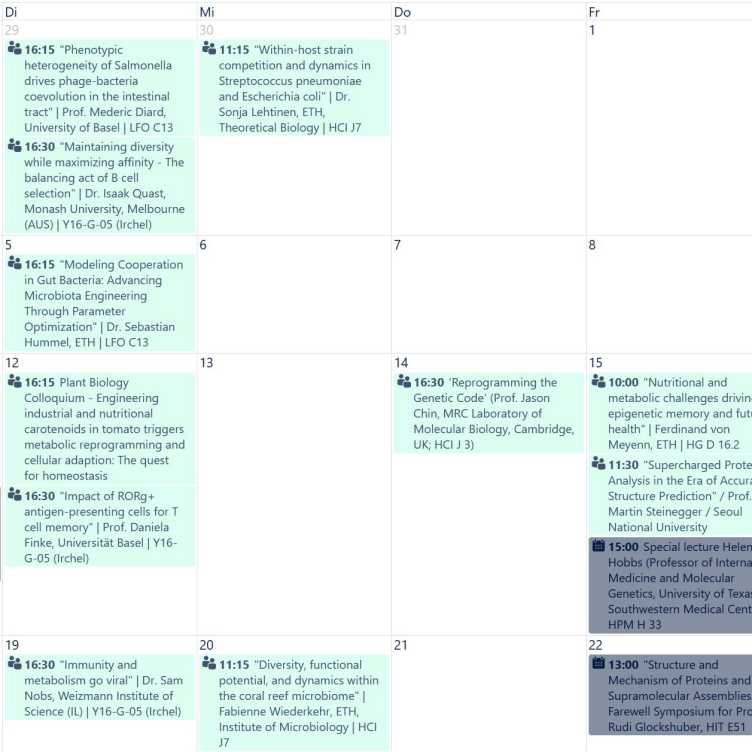
The D-BIOL seminar calendar provides information on seminars, symposia and promotional examinations that take place in the D-BIOL (access for members of the D-BIOL via nethz-login only).
ETH Biology Lectures

Every year an institute of the D-BIOL invites a high-profile speaker to the so-called 'ETH Biology Lecture'. Name, lecture title and research area of the speakers of the last years are listed here.
Department conferences

The dates of D-BIOL conferences (department conference, professor conference, grading conference, etc.) can be found here.
Bachelor and Master ceremonies

The date for the next Bachelor ceremony is 24 October 2025, the one for the Master ceremony is 12 December 2025.
"Your Future in Biology"

34 biologists present their careers in 3-minute presentations as part of "Your Future in Biology". All speakers, the videos of their presentations and the wide range of career opportunities can be found external page here.
