ATP binding allosterically drive DNA binding specificity of a primase
Primases have a fundamental role in DNA replication. They synthesize a primer that is then extended by DNA polymerases. Archaeoeukaryotic primases require for synthesis a catalytic and an accessory domain, the exact contribution of the latter being unresolved. A recent "Cell" paper by the Allain group (IMBB) sheds light on the underlying mechanism.
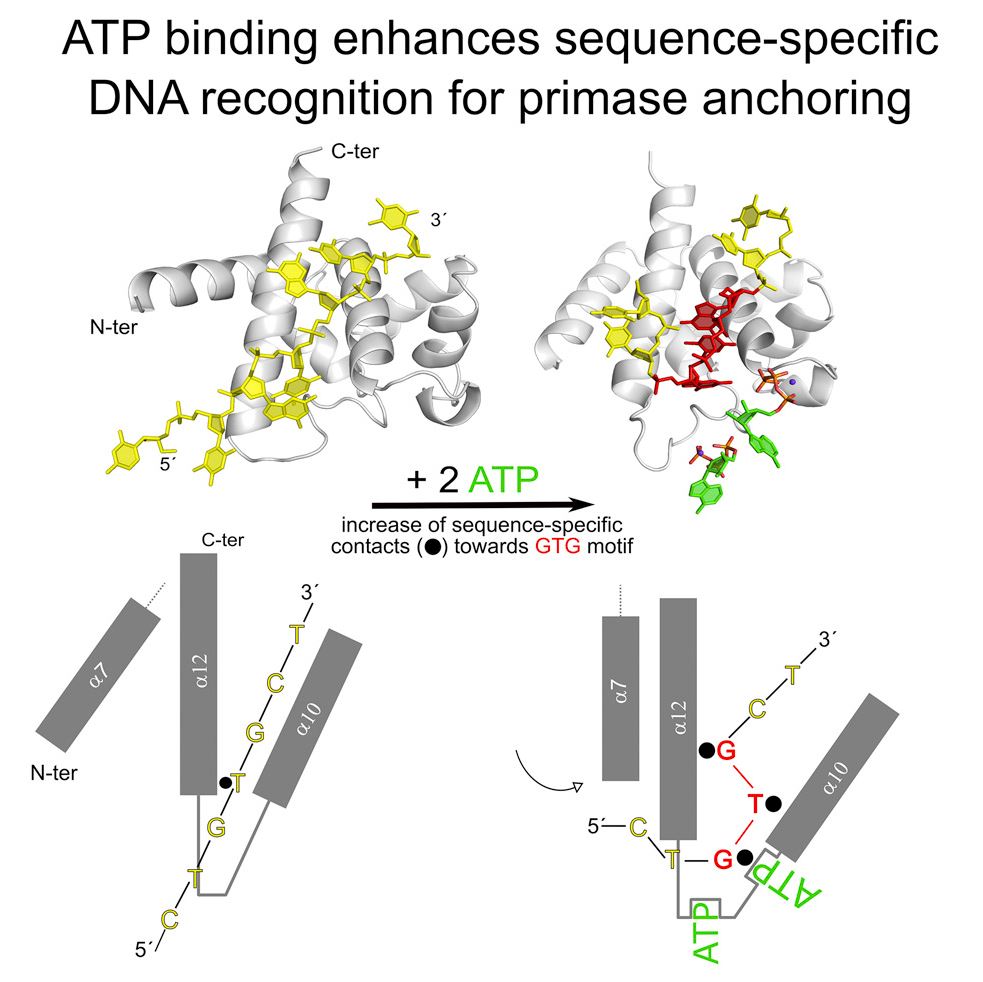
For the pRN1 archaeal primase, this domain is a 115-amino acid helix bundle domain (HBD). The Allain group's structural investigations of this small HBD by liquid- and solid-state nuclear magnetic resonance (NMR) revealed that only the HBD binds the DNA template. DNA binding becomes sequence-specific after a major allosteric change in the HBD, triggered by the binding of two nucleotide triphosphates. The spatial proximity of the two nucleotides and the DNA template in the quaternary structure of the HBD strongly suggests that this small domain brings together the substrates to prepare the first catalytic step of primer synthesis. This efficient mechanism is likely general for all archaeoeukaryotic primases.
external page Link to the publication in "Cell".