Structural insights into an atypical secretory pathway kinase crucial for Toxoplasma gondii invasion
The recent Nature Communications paper by the groups of Dominique Soldati-Favre (UNIGE), Adrian Hehl (UZH) and Volodymyr Korkhov (IMBB, ETHZ & PSI) describes the structure and function study of RON13, a kinase that controls host cell invasion by Toxoplasma gondii.
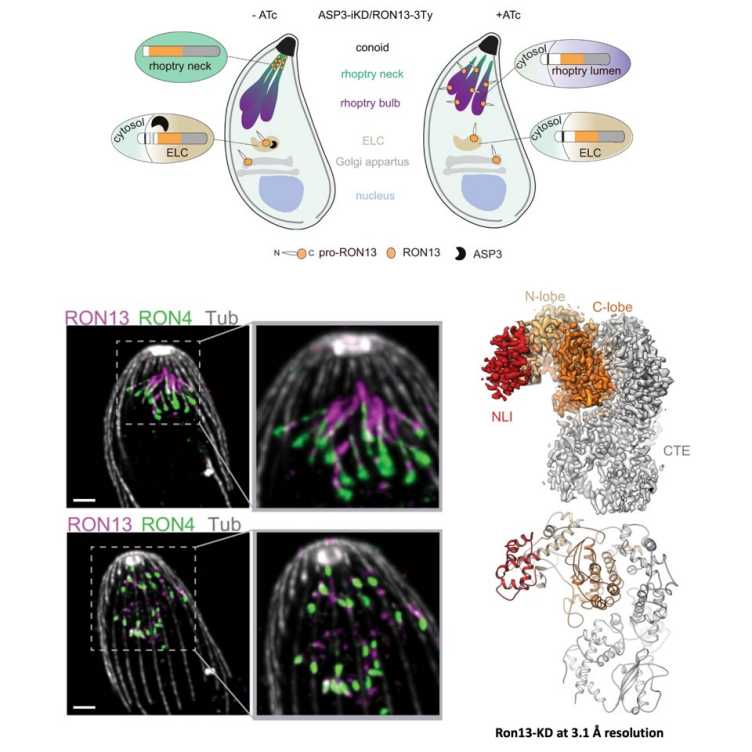
The recent Nature Communications paper by the groups of Dominique Soldati-Favre (UNIGE), Adrian Hehl (UZH) and Volodymyr Korkhov (IMBB, ETHZ & PSI) describes the structure and function study of RON13, a kinase that controls host cell invasion by Toxoplasma gondii.
Toxoplasma gondii, the parasite responsible for toxoplasmosis, is capable of infecting almost all cell types. It is estimated that up to 30% of the world's population is chronically infected, the vast majority asymptomatically. However, toxoplasmosis is a common opportunistic infection with high mortality in individuals who are immunocompromised and congenital infection can result in severe developmental pathology in the unborn child. Like the other members of the large phylum of Apicomplexa, T. gondii is an obligate intracellular parasite which, to survive, must absolutely penetrate its host's cells and hijack their functions to its own advantage. Understanding how the parasite manages to enter host cells offers new opportunities to develop more effective prevention and control strategies than those currently available. A team including the groups of Prof. Dominique Soldati-Favre (University of Geneva), Prof. Adrian Hehl (University of Zurich) and Prof. Volodymyr Korkhov (Paul Scherrer Institute and ETH Zurich), have identified the key role of RON13, a protein located in the rhoptry secretory organelle of the parasite, which is essential for the invasion process. The three-dimensional structure and the site of action of this enzyme are atypical, thus offering the possibility of designing specific inhibitors to stop the infection.
Link to the paper in external page Nature Communications.