Base editing as a potential cure for Fanconi anemia
A recent collaborative effort of the Corn Group (IMHS) and Paula Rio’s lab (CIEMAT, Madrid) has been published in "Nature Communications". The authors found that base editing strategy can be used for efficient restoration of function in bone marrow stem cells from Fanconi Anemia patients.
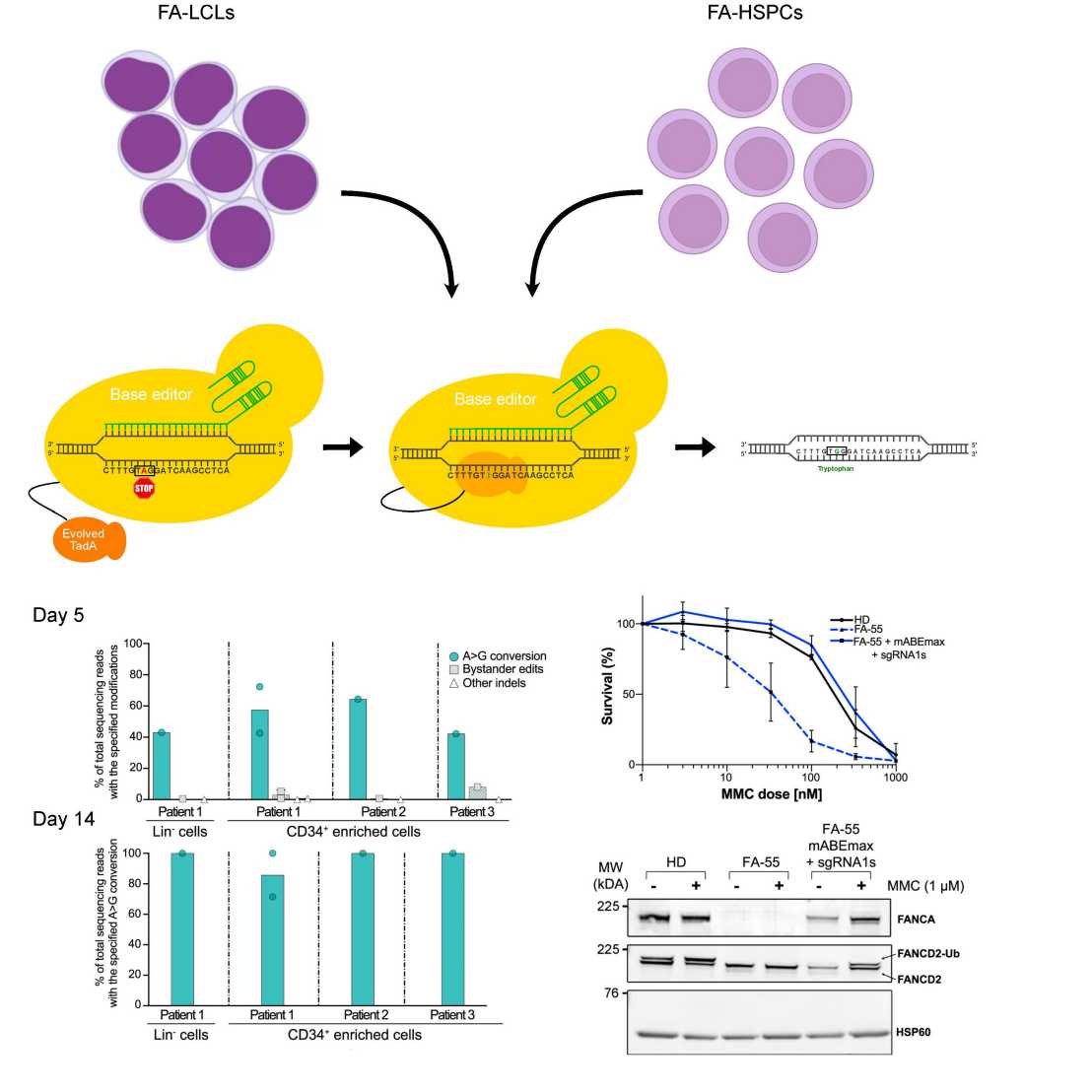
Cas-mediated genome editing technology holds great promise as a curative treatment for a number of genetic diseases. Conventional CRISPR-Cas genome editing induces DNA damage (double stranded DNA breaks) and relies on the cellular DNA repair system to yield the desired repair outcome. However, in Fanconi Anemia (FA), a genetic disorder associated with bone marrow failure and cancer predisposition, DNA repair is defective due to the gene mutations causing the disorder, preventing the use of editing strategies such as homology directed repair (HDR). Is it possible to avoid DNA cleavage, and instead use recently developed genome editing systems such as base editing? Can base editing reverse the effects of FA mutations? In this proof-of-concept study, the authors find that base editing can indeed restore the function of FA bone marrow stem cells. The team went through cycles of optimization for the conditions (base editor construct, vector type, guide RNA format, delivery) in cell lines from multiple FA patients. The developed approach effectively corrected FA mutations in both patient-derived cell lines and bone marrow stem cells from FA patients, leading to restored FANCA expression and functional FA pathway and phenotypic resistance to crosslinking agents.
Altogether, this work highlights base editors as a feasible editing strategy in FA and brings us one step closer to the future clinical implementation of base editing not only in FA, but also in other genetic diseases.
Link to the paper in "external page Nature Communications".