The dark side of fluorescent protein tagging – the impact of protein tags on biomolecular condensation
A recent paper by the Weis group (IBC) raises concerns about biomolecular condensation experiments with tagged proteins highlighting the need for caution when interpreting them. Focusing on the P-body protein Dhh1 they show that fluorescent proteins can dramatically alter condensate properties in vivo and in vitro.
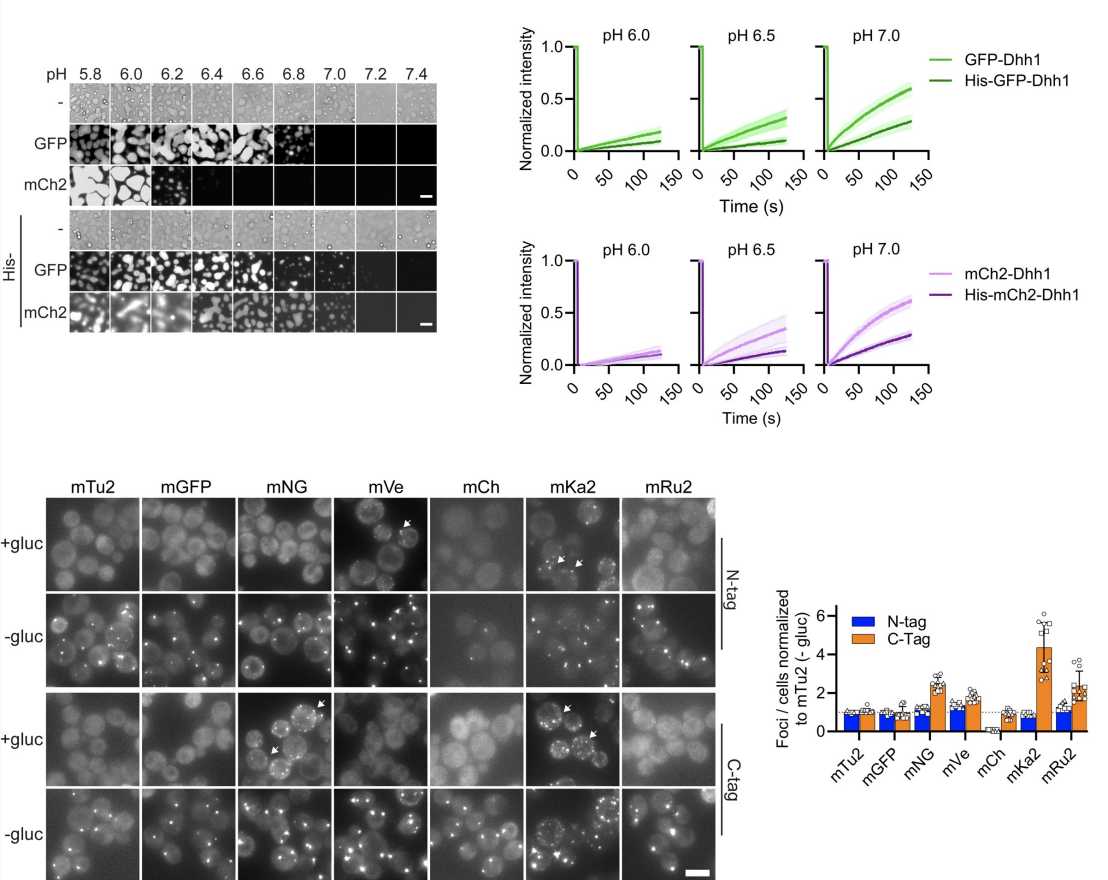
Fluorescent protein tags have been extensively used to study the formation and the properties of biomolecular condensates in vitro and in vivo, but there is evidence that tags may alter the condensation properties of proteins. In this study, the authors carefully assess the effects of protein tags on the yeast DEAD-box ATPase Dhh1, a central regulator of processing bodies (P-bodies), which are biomolecular condensates involved in mRNA metabolism. They show that fluorescent tags as well as a poly-histidine tag greatly affect Dhh1 condensation in vitro and lead to condensates with different dynamic properties. Tagging of Dhh1 with various fluorescent proteins in vivo alters the number of P-bodies upon glucose starvation and some tags even show constitutive P-bodies in non-stressed cells. These data raise concerns about the accuracy of tagged protein condensation experiments, highlighting the need for caution when interpreting the results.
Link to the paper in external page "Molecular Biology of the Cell".