How lipid membranes trigger protein aggregation in Parkinson’s—and how to stop it
A new PNAS study by the Michaels group (IBC) uncovers how lipid membranes trigger toxic α-synuclein aggregation and shows how small molecules can inhibit this process.
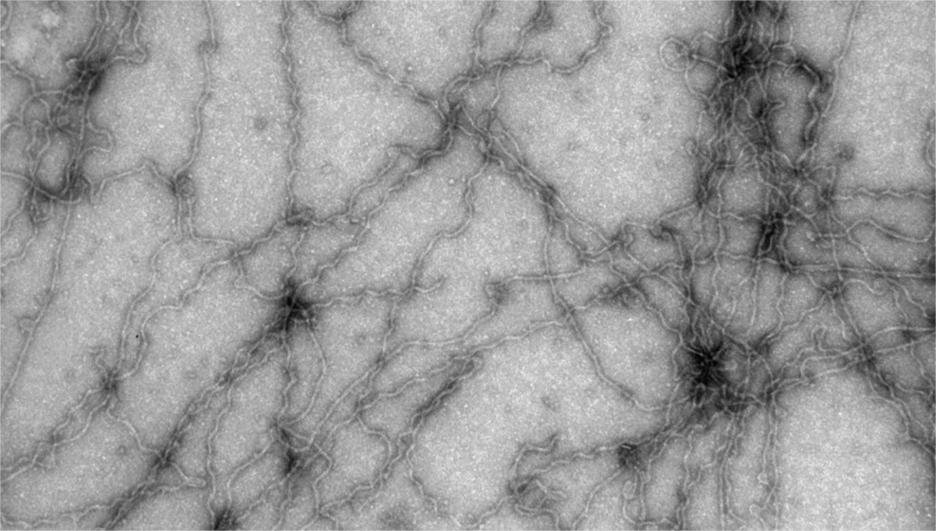
Parkinson’s disease affects over 10 million people worldwide, with numbers expected to rise sharply in coming decades. A key feature of the disease is the aggregation of the protein α-synuclein into toxic amyloid fibrils. These aggregates form not just from protein alone, but through interactions with lipid membranes—an important but poorly understood trigger.
In this PNAS study, researchers from the Michaels group at ETH Zurich, with collaborators from Cambridge and Copenhagen, developed a global kinetic model that quantitatively predicts how α-synuclein aggregates on lipid membranes. The model reveals that aggregation occurs via a two-step process: lipid surfaces first promote the formation of intermediate oligomers, which then convert into mature fibrillar coaggregates with lipids embedded.
Most importantly, the model enables the precise identification of which molecular steps during α-synuclein aggregation can be targeted for therapeutic intervention. The team used their framework to understand the action of squalamine, a naturally derived molecule that inhibits α-synuclein aggregation by blocking the lipid-induced nucleation step. These insights pave the way for rational design of drugs that target the early stages of protein aggregation at biological membranes, with the aim of potentially preventing or slowing down Parkinson’s progression.
Link to the paper in external page "PNAS".