Ubinuclein 2 is essential for mouse development and functions in X chromosome inactivation
A new study in PLoS Genetics by the Wutz group (IMHS) characterizes the two Ubinuclein genes in mouse development. While mutation of Ubinuclein 1 causes no overt phenotype, Ubinuclein 2 is shown to be critical for embryogenesis with a function in X chromosome inactivation in female embryos advancing our understanding of mammalian dosage compensation.
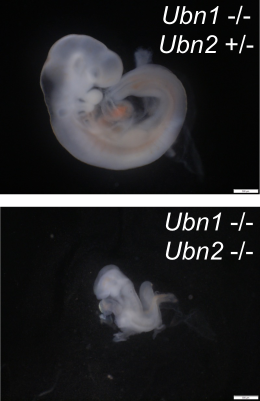
Assembly of eukaryotic genomes into a nucleosomal structure requires deposition of histone proteins on the DNA. This process involves distinct mechanisms that are wither dependent or independent of DNA replication. The evolutionary conserved HIRA complex deposits histone H3.3 independent of DNA replication within the chromatin of active gene loci and regulatory regions. Specific recognition of histone H3.3 is mediated by a Ubinuclein subunit. In mice, Ubinucleins are encoded by two genes, Ubn1 and Ubn2. Monfort et al. characterize these genes in mice and show that Ubn1 is not essential. Homozygous Ubn1 mutant mice can be obtained and are healthy and fertile. In contrast, the mutation of Ubn2 causes embryonic lethality with incomplete penetrance, and combined mutations in both Ubinuclein genes lead to lethality with complete penetrance at an early embryonic stage.
The study also reveals a female specific function in embryogenesis. Molecular characterization in mouse embryonic stem cells shows that HIRA complex function is required for establishing gene silencing on the inactive X chromosome. Without Ubinuclein proteins a switch from acetyl to tri-methyl modified histone H3 lysine 27 at X-linked genes is not observed during the initiation of X chromosome inactivation pinpointing a molecular event that is dependent on HIRA complex function. Advancing the understanding of the physiologic role of HIRA complex genes in development and dosage compensation is also relevant for human diseases that are associated with histone H3.3 mutations.
Link to the paper in external page "PLoS Genetics"