The Atg1 kinase complex orchestrates spatial and temporal control of autophagosome formation
A recent paper by the Peter and Aebersold groups reports that the Atg1 kinase complex functions at multiple levels during autophagy. Upon autophagy induction, lipidated Atg8 activates the Atg1 kinase, which in turn phosphorylates Atg13 triggers its dissociation. Active Atg1 then travels along the growing autophagosome, where it inhibits two enzymes involved in Atg8 lipidation.
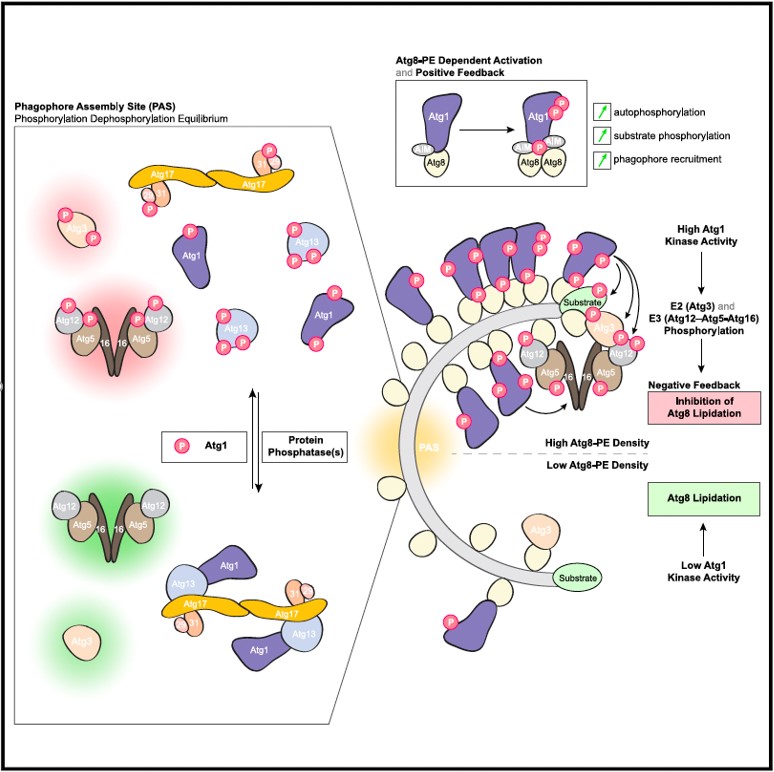
Autophagy is a conserved intracellular degradation pathway exerting various cytoprotective and homeostatic functions by using de novo double-membrane vesicle (autophagosome) formation to target a wide range of cytoplasmic material for vacuolar/lysosomal degradation. The Atg1 kinase is one of its key regulators, coordinating a complex signaling program to orchestrate autophagosome formation. Combining in vitro reconstitution and cell-based approaches, we demonstrate that Atg1 is activated by lipidated Atg8 (Atg8-PE), stimulating substrate phosphorylation along the growing autophagosomal membrane. Atg1-dependent phosphorylation of Atg13 triggers Atg1 complex dissociation, enabling rapid turnover of Atg1 complex subunits at the pre-autophagosomal structure (PAS). Moreover, Atg1 recruitment by Atg8-PE self-regulates Atg8-PE levels in the growing autophagosomal membrane by phosphorylating and thus inhibiting the Atg8-specific E2 and E3. Our work uncovers the molecular basis for positive and negative feedback imposed by Atg1 and how opposing phosphorylation and dephosphorylation events underlie the spatiotemporal regulation of autophagy.
Link to the paper in external page Molecular Cell