New methods and applications of cross-linking mass spectrometry for structural biology
Three recent publications with contributions of the Leitner group (IMSB) in Analytical Chemistry, ACS Measurement Science Au and Nature emphasize the role of mass spectrometry to study the structure of proteins and protein complexes as well as protein-RNA interactions.
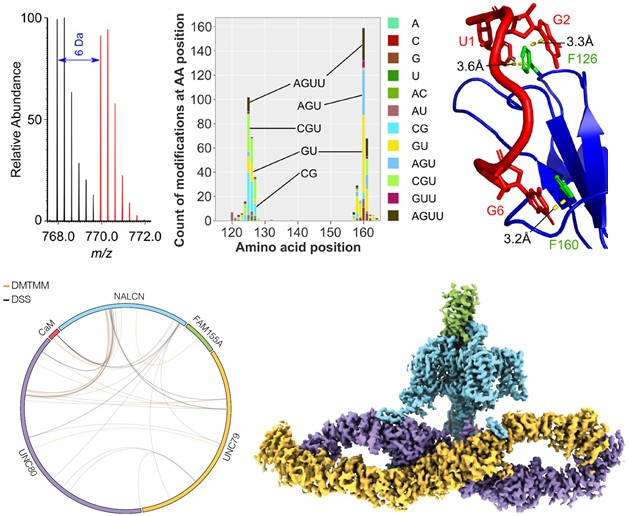
Chemical cross-linking is one of a group of structural mass spectrometry/structural proteomics methods that has found widespread use in proteomics, structural biology and systems biology research. The Leitner group at IMSB develops cross-linking methods and applies them to study protein-protein and protein-RNA interactions. Three recent publications highlight the potential of this technique.
In Analytical Chemistry, work led by former postdoctoral researcher Michael Götze describes a new approach to introduce stable isotope labels for the CLIR-MS workflow, a protein-RNA cross-linking method that has been developed in collaboration with the Allain group at IBC. The method is easy to set up and expands the range of applications for the study of ribonucleoprotein assemblies. This research was carried out as part of a project funded by the ETH Strategic Focus Area “Personalized Health and Related Technologies”.
In ACS Measurement Science Au, former postdoctoral researcher Esben Trabjerg and Arend Keller (now PhD student at D-HEST) demonstrate that it is also possible to perform protein cross-linking with widely used succinimide-based reagents at low pH. This way, the technique can also be applied to intracellular compartments with low local pH, such as lysosomes.
Very recently, a publication in Nature highlighted the use of cross-linking mass spectrometry for the structural analysis of large protein complexes. The structure of the NALCN ion channel complex was solved by cryo-electron microscopy and its function was assessed by electrophysiological methods. In this collaboration with colleagues from Genentech and the University of Copenhagen, among others, cross-linking provided preliminary data for delineating the arrangement of complex subunits, and confirmed that calmodulin, which co-purified with NALCN subunits, binds specifically at a particular location within the complex and may have a functional role.
Link to the paper in external page Analytical Chemistry
Link to the paper in external page ACS Measurement Science Au
Link to the paper in external page Nature