TMED2 restricts SMO trafficking from the ER-Golgi
A recent “PLOS Biology” paper by Di Minin et al. (Wutz group at IMHS) identifies the Golgi protein TMED2 as a novel modulator of the Hedgehog pathway and suggests the ER-Golgi trafficking as a crucial process in the activation of the signalling cascade.
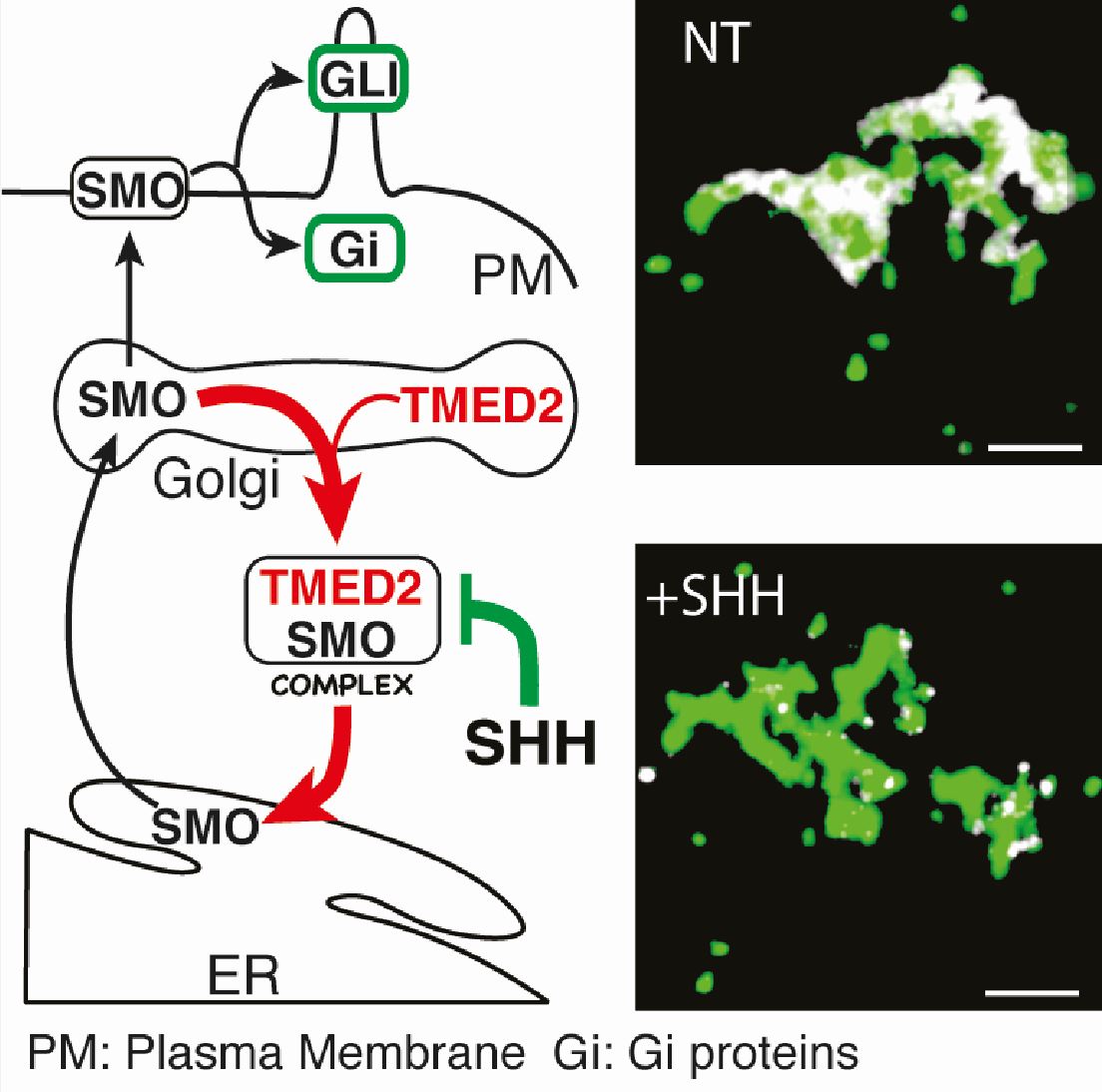
Hedgehog (HH) signalling is important for embryo development and regulating cell stem differentiation. HH signals are transduced by the G-protein coupled receptor Smoothened (SMO) and elicit transcriptional and transcriptional independent responses. SMO is the target of multiple drugs used in clinics for counteracting tumour progression. Its sub-cellular distribution has been the focus of multiple debates and considered central to decipher HH activation mechanisms.
Research in the Wutz lab in collaboration with the Jerome-Majewska (McGilli University, Canada) and Roelink (University of California-Berkley, USA) labs uncovered a new mechanism of SMO trafficking from the ER-Golgi apparatus in the regulation of HH signalling. Through a forward genetic screen in haploid embryonic stem cells, the Golgi protein TMED2 was identified as a factor that binds SMO and regulates its sub-cellular distribution. 3D-STORM super-resolution microscopy allowed to position SMO in the ER-Golgi compartments and show that TMED2 prevents its trafficking to the plasma membrane. The interaction of SMO and TMED2 is regulated by HH signals. Release of SMO from TMED2 is one of the earliest steps of the activation of the signalling cascade.
The new results provide an entry point for unravelling the enigmatic signal transduction mechanism from the HH receptor Patched to Smoothened. Future investigation of the SMO-TMED2 complex and the ER-Golgi trafficking will open opportunities for targeting the HH pathway in diseases.
Link to the paper in "external page PLOS Biology"