Shedding new light on the mechanism of individual cell memory, using yeast as a model
In “Current Biology”, the Barral group at IBC, in collaboration with the Caudron group at the Queen Mary University in London, and the Saarikangas group at the University of Helsinki, and the Hilvert group at D-CHAB has brought to light two new pieces of information in the Whi3 mnemon puzzle.
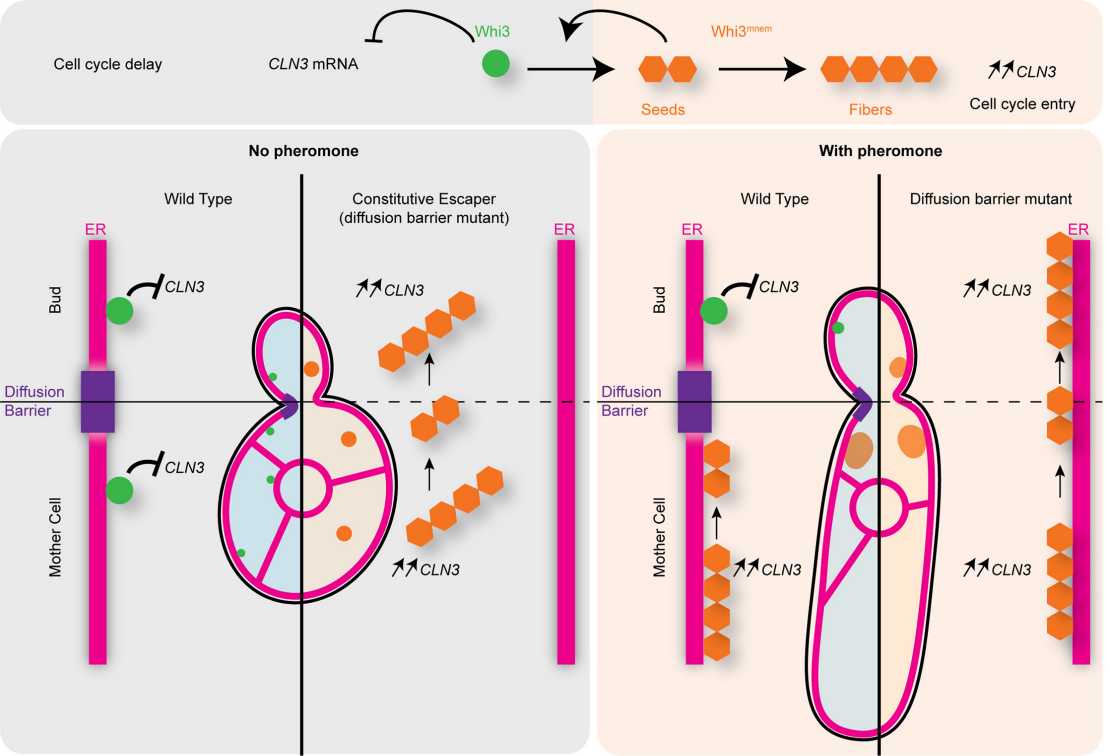
Whi3 is a mnemon (Whi3mnem), or, in other words, a protein that stores information in individual cells by forming stable super-assemblies. This memory state is then inherited by only one individual daughter cell at mitosis (individual memory). For example, when facing an uncommitted mating partner, budding yeast cells coalesce the G1/S inhibitor Whi3 into a dominant mnemon super-assembly that drives escape from mating pheromone response. This mnemon state remains stable in the mother cell for many division cycles without being passed to its daughters. How cells maintain and partition the Whi3mnem state at mitosis was until now largely unknown.
In this study, the researchers show that Whi3mnem associates with the endoplasmic reticulum (ER) membrane and is confined to the mother cell by ER-lateral diffusion barriers at the bud neck. Furthermore, when these barriers are lifted, Whi3mnem, which forms self-templating fibrils in vitro, propagates to its entire progeny like a prion, making memory collective.
Based on this evidence, the authors conclude that the self-templating prion-like behavior of Whi3mnem is needed for the persistence of memory whereas its association with the ER prevents its infectious propagation to the daughter cells. These findings provide a first mechanism for how cells can form and maintain individual memory.
A question naturally follows from these results: Are persistence and confinement conserved mechanisms for encoding individual memory across organisms? These findings could have important implications in pathological contexts: In fact, age-related confinement defects may unleash the prion-like behavior of mnemons, allowing them to spread in tissues and to promote phenotypes very similar to those observed in neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Link to the paper in Current Biology