Mre11-Rad50 oligomerization promotes DNA double-strand break repair
A recent “Nature Communications” paper by the Peter and Cejka groups (IBC, ETH/IRB) reveals how the oligomerization of a conserved protein complex is critical for DNA damage signaling, DNA repair and telomere maintenance, with potential implications for cancer predisposition and chemoresistance.
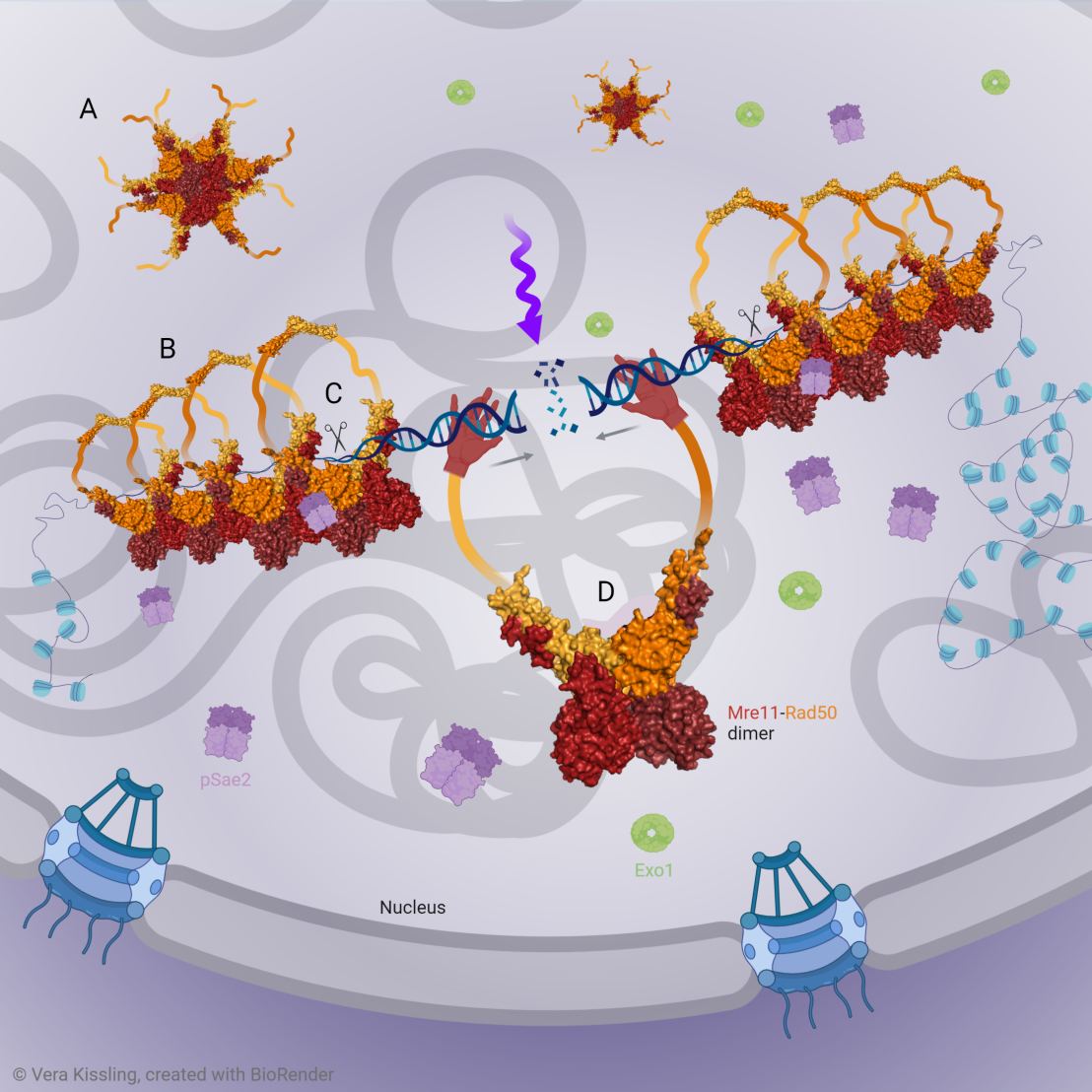
The integrity of cellular DNA is essential for human health, as the accumulation of DNA damage is linked to the development of cancer and neurodegenerative diseases. Of all DNA lesions, DNA double-strand breaks (DSBs) have the most severe consequences. A key player in the recognition, signaling and nucleolytic end processing of DSBs for subsequent repair is the conserved Mre11-Rad50 (MR) protein complex. Although it is known for two decades that MR forms nuclear foci in eukaryotic cells that accompany DNA repair, the molecular mechanisms underlying foci formation and their functional importance for DSB repair remained unclear.
This paper reveals that MR dimers oligomerize to form nucleolytically active assemblies in solution and on DNA via a conserved beta-sheet in the head domain of Rad50. Coupling DNA repair pathway reconstitution with TEM imaging, Kissling et al. show that MR oligomerization is required for its endo- but not for its exonuclease function in DNA end processing. Mutational analysis identified specific beta-sheet mutations that enhance or disrupt MR oligomerization. Studies of these Rad50 mutants in yeast cells confirmed that beta-sheet driven MR oligomerization is a prerequisite for MR foci formation, DNA damage checkpoint signaling, proper DSB repair and maintenance of normal telomere length. Interestingly, oligomerization-disrupting mutations are found in persons predisposed to hereditary BRCA1/2-PALB2-cancers and may play a role in tumor chemoresistance.
Link to the paper in "external page Nature Communications"