Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
A combination of genome mining, analytical and computational chemistry, total synthesis, molecular biology enabled the discovery of novel potent cytotoxins, janustatins. This collaborative effort of the Piel and Oxenius groups (D-BIOL, Institute of Microbiology), with the Carreira group (D-CHAB, Laboratory of Organic Chemistry) as well as Japanese and US researchers was recently published in Nature Chemistry.
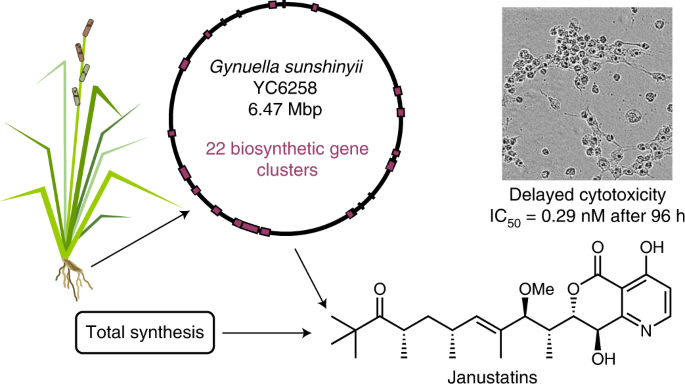
Host-associated bacteria are increasingly being recognized as underexplored sources of bioactive natural products with unprecedented chemical scaffolds. A recently identified example is the plant-root-associated marine bacterium Gynuella sunshinyii of the chemically underexplored order Oceanospirillales. Its genome contains at least 22 biosynthetic gene clusters, suggesting a rich and mostly uncharacterized specialized metabolism.
Here, in silico chemical prediction of a non-canonical polyketide synthase cluster has led to the discovery of janustatins, structurally unprecedented polyketide alkaloids with potent cytotoxicity that are produced in minute quantities. A combination of MS and two-dimensional NMR experiments, density functional theory calculations of 13C chemical shifts and semiquantitative interpretation of transverse rotating-frame Overhauser effect spectroscopy data were conducted to determine the relative configuration, which enabled the total synthesis of both enantiomers and assignment of the absolute configuration. Janustatins feature a previously unknown pyridodihydropyranone heterocycle and an unusual biological activity consisting of delayed, synchronized cell death at subnanomolar concentrations.
Link to the paper in external page Nature Chemistry