Punching a hole in the nuclear membrane
A recent eLife paper from the Weis group, in collaboration with the Medalia, Onischenko and Antonin labs, suggests a role for the protein Brl1 in the enigmatic membrane fusion event that leads to a functional nucleocytoplasmic transport channel during nuclear pore complex biogenesis.
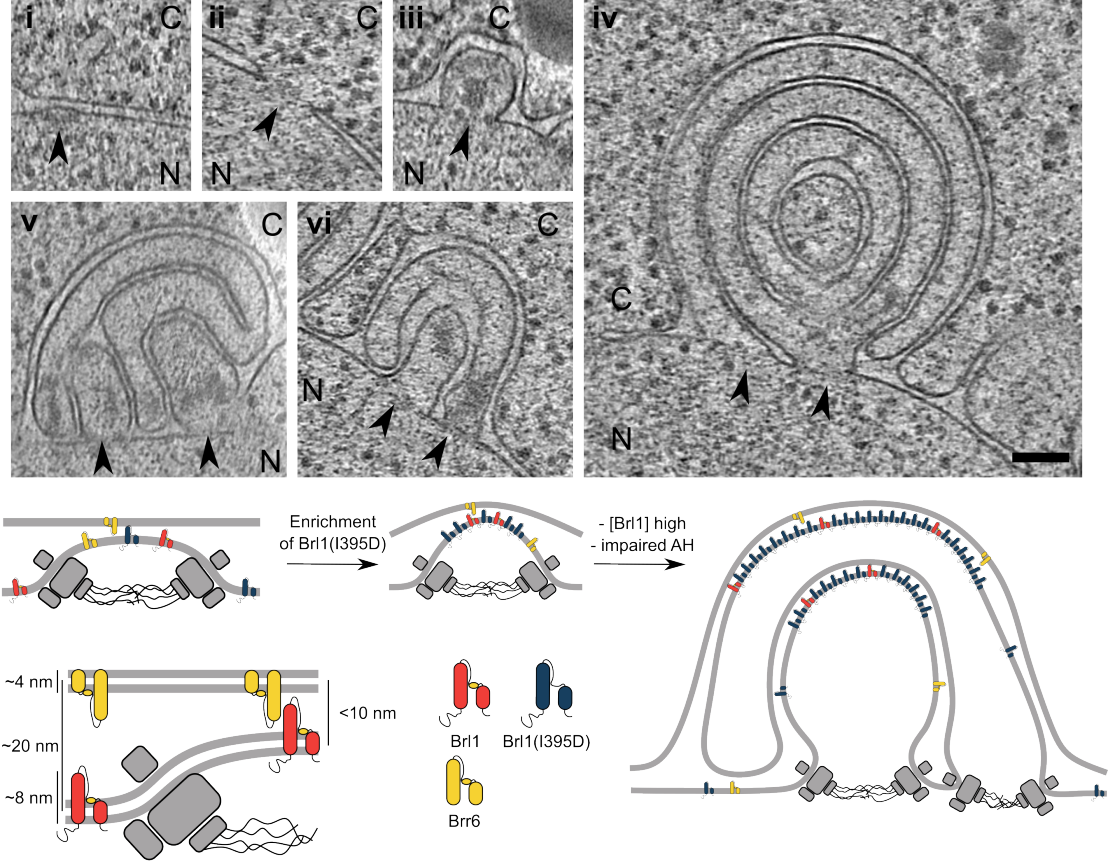
The yeast nuclear pore complex (NPC) is a massive, ~50 MDa structure that spans the double membrane of the nucleus and facilitates the exchange of macromolecules between the nucleus and cytoplasm. Despite its enormous size, very few trans-acting factors have been implicated in its biogenesis. Furthermore, little is known about how the NPC is inserted into the double lipid bilayer of the nuclear envelope, or what triggers fusion of the outer and inner nuclear membranes during NPC assembly.
In this work, the Weis group identified the transmembrane protein Brl1 as an NPC assembly factor required for nuclear membrane fusion in budding yeast. Depleting the protein led to unfused extrusions of the nuclear envelope, suggesting a block in NPC biogenesis during membrane insertion. The group went on to identify an amphipathic helix motif in Brl1 which binds preferentially to highly curved membranes and is essential for cell viability. Cryo-electron tomography analysis revealed that, in mutants of the amphipathic helix, large, onion-like herniations form at the nuclear envelope, with stalled NPC assembly intermediates visible at their base.
Taken together, this work shows that Brl1 is required for the critical “hole-punch” step in NPC biogenesis, and brings us one step closer to unravelling the mechanisms involved in the maturation of this highly complex cellular structure.
Link to the paper in external page eLife