Glycan recognition and reaction priming of eukaryotic oligosaccharyltransferase
A recent article in the journal "Nature Communications" by the Locher group (IMBB) in collaboration with the Aebi (IMB) and Reymond (Uni Bern) groups revealed how eukaryotic oligosaccharyltransferase enzymes recognize their glycan donor to modify acceptor proteins.
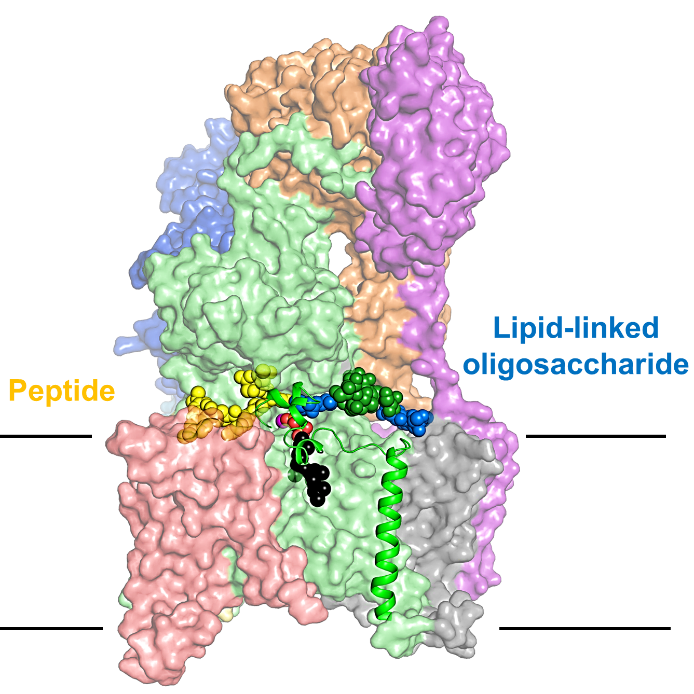
N-linked protein glycosylation is one of the most common post-translational modifications found in nature. N-glycans play essential roles in protein folding, trafficking, and cell-cell communication. The central step of this process is catalyzed by the membrane protein complex oligosaccharyltransferase (OST), which transfers a pre-assembled oligosaccharide from a lipid carrier to acceptor proteins in the lumen of the endoplasmic reticulum. Recognition of acceptor proteins by OST was well understood, in great part thanks to previous work of the Locher and Aebi groups at ETH in collaboration with the Reymond group at Uni Bern, but the molecular basis of glycan recognition had remained unknown.
Ramírez AS, et al., used a chemo-enzymatic approach to generate a donor substrate analog of OST that carries the native oligosaccharide. They obtained cryo-electron microscopy (cryo-EM) structures of S. cerevisiae OST in distinct functional states, revealing that two non-catalytic subunits of the OST complex bind the three terminal glucoses (Glc3) of the glycan donor, which represent the final steps of oligosaccharide assembly. Recognition of the Glc3 moiety explains the high specificity of OST towards the fully assembled oligosaccharide in vivo. Their results explain how one of the most important post-translational modifications occur, and will benefit further investigation on the design of OST inhibitors, which have been proven useful in the development of antiviral drugs and as chemotherapeutic helpers.
Link to the paper in "external page Nature Communications"