Non-enzymatic acetylation inhibits glycolytic enzymes in E. coli
A recent "Cell Reports" paper by the Sauer group (IMSB) quantified non-enzymatic acetylation in central metabolism by intact protein mass spectrometry, demonstrating a novel regulation of glycolytic flux through acetylphosphate-dependent acetylation.
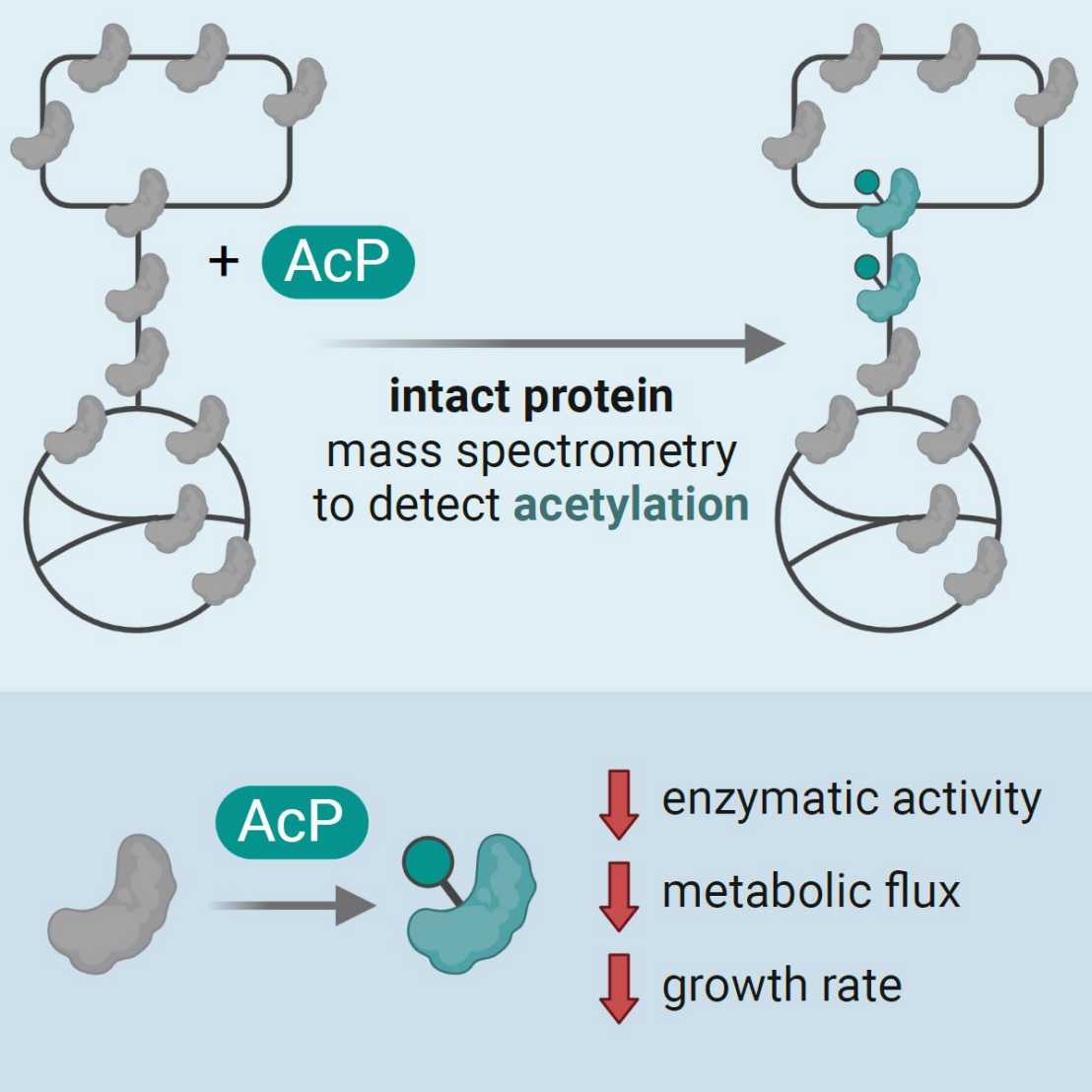
We quantify, for the first time, the stoichiometry of non-enzymatic protein acetylation driven by the cellular metabolite acetyl-phosphate on central metabolic enzymes in the bacterium E. coli. The extent of acetylation allows us to assess whether a given modification reaches a stoichiometry sufficient to noticeably inhibit protein function in vivo. While the majority of previously reported acetylation events remain at very low stoichiometry, we demonstrate two specific targets of chemical acetylation that inhibit the glycolytic enzymes GpmA and GapA under physiological conditions, thus providing evidence for an additional, non-enzymatically catalyzed layer of glycolytic flux regulation.
Link to the paper in "external page Cell Reports".