Glucose stress causes mRNA retention in nuclear Nab2 condensates
A recent "Cell Reports" paper by the Weis group (IBC) shows that nutrient stress causes the yeast nuclear poly(A)-binding protein Nab2 to form mRNA-containing condensate-like structures in the nucleus. The work suggests that this regulates mRNA export and gene expression during stress.
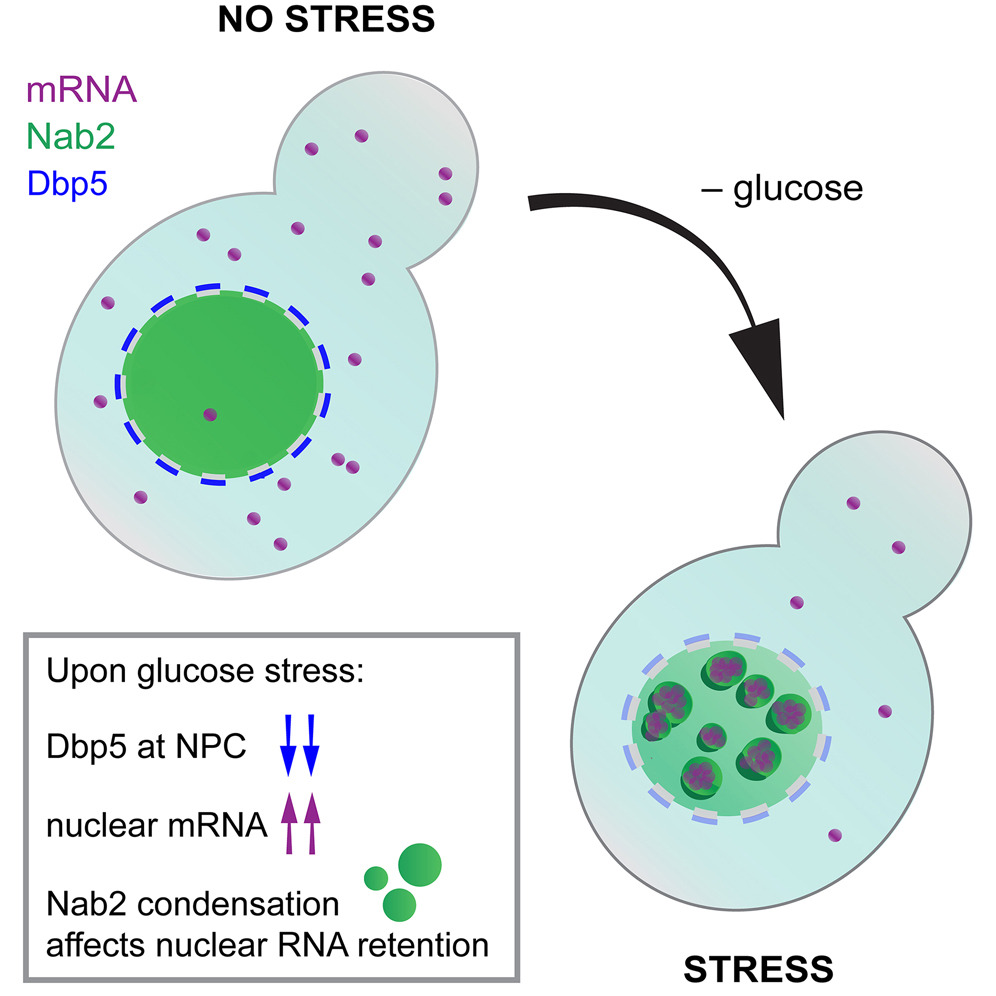
Subcellular compartmentalization is a fundamental principle that allows eukaryotic cells to regulate their gene expression. For example, the nucleus and cytoplasm are separated by double membrane embedded channels, known as nuclear pore complexes (NPCs), which regulate the selective transport of macromolecules into and out of the nucleus. Biomolecular condensates are membraneless organelles that provide an alternative mechanism for organizing cellular components and regulating molecular interactions. In yeast, these condensates often form upon stress, as in the case of P bodies and stress granules in the cytoplasm.
In this work, Heinrich et al. show that blocking mRNA export by removing glucose or depleting the DEAD-box ATPase Dbp5 from the NPC results in the formation of nuclear foci that contain mRNA and the mRNA export factor Nab2. These nuclear foci resemble biomolecular condensates and can also form in vitro in the presence of RNA. Mutants of Nab2 that affect the size, number and biophysical properties of the condensates also affect the degree of mRNA retention in the nucleus: impairing the formation of Nab2 foci results in increased mRNA export, while enhancing the condensation phenotype increases mRNA retention. These results suggest that Nab2 plays a role in regulating gene expression during stress by forming condensates that prevent the export of bulk mRNA from the nucleus.
Link to the paper in external page "Cell Reports".