Mitotic chromosome condensation resets chromatin to safeguard transcriptional homeostasis during interphase
In a collaborative paper in PNAS, Yves Barral with Jorrit Enserink and the Chymkowitch group in Norway shows that the effects on transcription of chromosome condensation during mitosis, is not just a passive phenomenon, but has strong biological significance: It resets the transcriptome to protect cells from uncontrolled transcriptional drift during the following interphase. Thereby, it contributes to the homeostatic control of gene expression regulation.
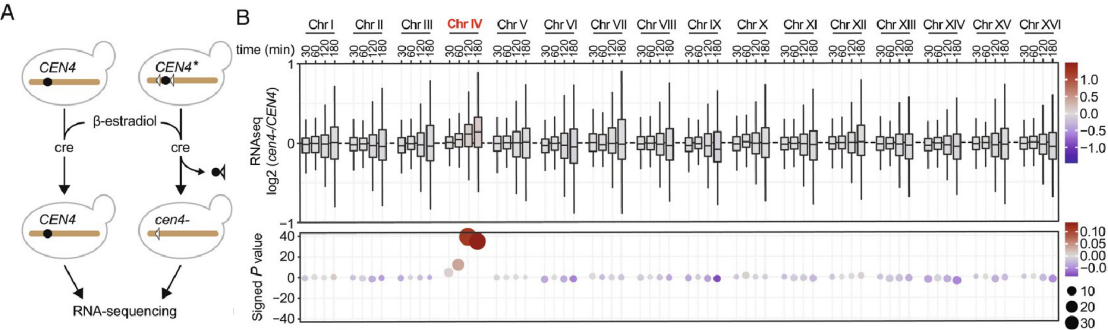
Mitotic entry correlates with the condensation of the chromosomes, changes in histone modifications, exclusion of transcription factors from DNA, and the broad downregulation of transcription. However, whether mitotic condensation influences transcription in the subsequent interphase is unknown.
In their collaborative paper now published in PNAS, Yves Barral, Jorrit Enserink and the group of Pierre Chymkowitch in Norway show that preventing one chromosome to condense during mitosis causes it to fail resetting of transcription. Rather, in the following interphase, the affected chromosome recruits unusually high levels of the transcription machinery, resulting in abnormally high expression levels of the genes it carries, including various transcription factors. This subsequently causes the activation of inducible transcriptional programs across the genome even in the absence of the relevant stimuli. For example, even in absence of galactose the GAL genes start being expressed at high levels throughout the genome due to lack of repression of their master regulator on the affected chromosome. In contrast, gene expression is overall dampened on all other chromosomes due to titration mainly of the RNA polymerase II by the decondensed chromosome. Thus, mitotic chromosome condensation exerts stringent control on interphase gene expression to ensure the maintenance of basic cellular functions and cell identity across cell divisions.
The effect of chromosome condensation on epigenetic markers and on gene expression in the ensuing interphase could also be relevant for asymmetrically dividing cells, such as stem cells, in which resetting gene expression programs might be crucial for stem cell renewal. The study reveals an unexpected mechanism by which cells prevent transcriptional drifting, providing new inroads for exploring how cells control their identity and homeostasis at the transcriptional level. It also reveals the importance for proper gene regulation of the RNA polymerase II being a limiting factor. These results may contribute to a better understanding of postmitotic aging and the etiology of diseases involving malfunctioning centromeres.
Link to the paper in external page "PNAS".