Cytoplasmic contractile injection systems mediate cell death in Streptomyces
In a collaborative paper published in "Nature Microbiology", the Pilhofer group (IMBB) and the Schlimpert group (John Innes Centre, UK) show that a cytoplasmic contractile injection system in the Gram-positive multicellular model organism Streptomyces coelicolor mediates cell death.
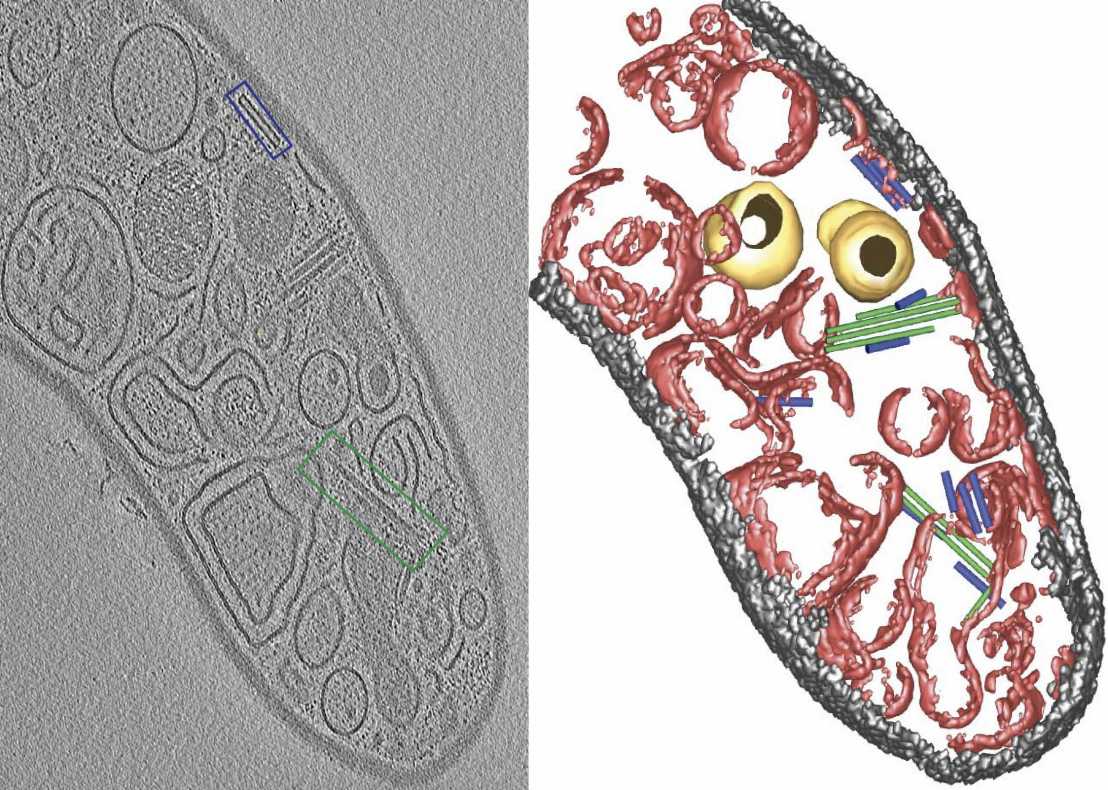
Contractile injection systems (CIS) are bacteriophage tail-like structures that function as spring-loaded nanosyringes to inject cargo into cells. Bacterial cells utilize these macromolecular assemblies to kill prey cells or manipulate target cells. Although CIS gene clusters are abundant in diverse bacterial phyla, these systems are poorly understood in Gram-positive organisms— bacteria with a thick cell wall.
In this recent study, the scientists structurally and functionally characterized CIS from Streptomyces coelicolor. These filamentous bacteria are abundant in soil and famous for their complex multicellular lifestyle. Furthermore, these organisms are significant for biotechnology and medicine because they produce important antibiotics and secondary metabolites.
In an integrative approach, the scientists employed a combination of cryo-electron tomography, single particle cryo-electron microscopy, time-lapse light microscopy imaging, and classical microbiological methods. Unlike most CIS that have been studied previously, CIS particles in S. coelicolor are free-floating in the cytoplasm and are not released into the surrounding medium. Notably, fired CIS were predominantly found in dead cells, suggesting that CIS could be involved in mediating death of the producing cell.
In order to test this hypothesis, the authors solved the structure of the contractile sheath of the CIS, which enabled them to genetically engineer a non-contractile mutant, as well as a fluorescently tagged version. Using these strains, Casu at el. were then able to set up assays that allowed them to determine the role of CIS under different stress conditions— for example, upon exposure to UV light or an antimicrobial peptide. Strikingly, cells that lacked a functional CIS were found to induce less cell death compared to the wildtype. This in turn affected the complex life cycle and resulted in significant differences in the timing of sporulation and secondary metabolite production. Finally, the authors set out to identify proteins that were loaded into the CIS. They identified three putative effectors that are likely released upon CIS contraction and mediate cell death via a yet unknown mechanism.
Whether or not programmed cell death plays an important role in the life cycle progression of Streptomyces has been a point of contention. The present study suggests that cellular suicide is indeed important for the differentiation of hyphae and the study also identified a mechanism by which this can be achieved. In support of this, CIS gene clusters are abundant and conserved among different Streptomyces species. Finally, the study provides a framework for studying intracellular roles of CIS, in contrast to the well-characterized, mainly antagonistic, intercellular functions of related systems.
Link to the paper in external page "Nature Microbiology".