Heterocomplex structure of a polyketide synthase component involved in modular backbone halogenation
A recent "Structure" paper by the Piel group (Institute of Microbiology) in collaboration with the Groll group (Technical University of Munich) demonstrates a novel protein pair responsible for halogenation of complex cytotoxic polyketides.
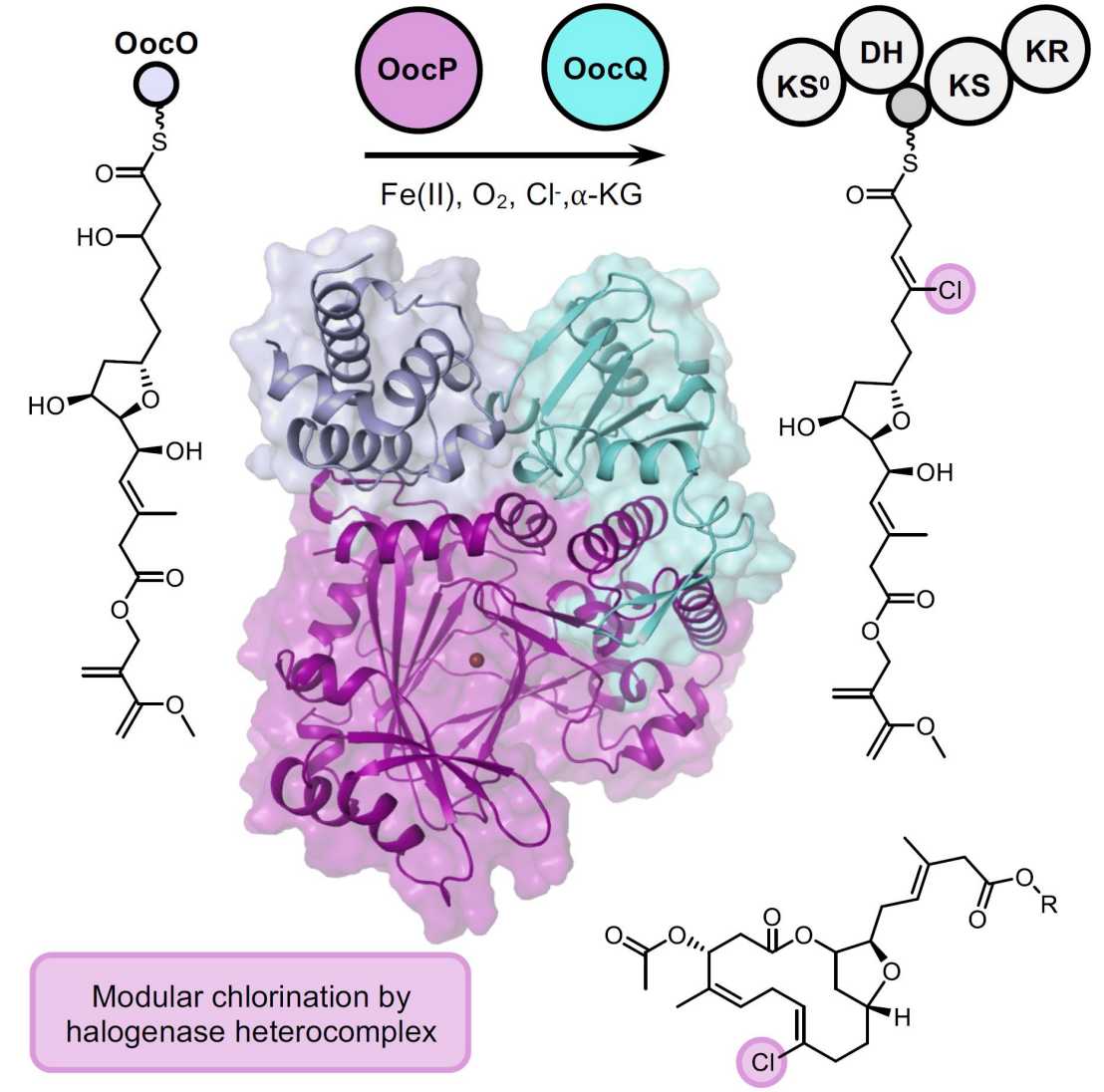
Members of the oocydin/haterumalide family of natural products display potent anti-oomycete and cytotoxic effects. These molecules are biosynthesized by multimodular enzymatic assembly lines termed trans-acyltransferase (trans-AT) polyketide synthases (PKSs), where a trans-acting AT selects the building blocks and loads them onto acyl carrier proteins (ACPs). Trans-AT PKSs feature an astonishing diversity of biosynthetic modules with integrated and trans-acting components.
The oocydin PKS is one of the biochemically most versatile enzymatic assembly lines characterized to date, with more than 10 modules installing a distinct chemical moiety during biosynthesis. One so far unparalleled biochemical transformation from this pathway is the introduction of a vinyl chloride moiety to a 21-carbon chain during polyketide elongation. The Fe(II)/ɑ-KG-dependent halogenase OocP from the oocydin pathway is so far the only known example of an enzyme halogenating an ACP-bound polyketide intermediate.
In the first instance of its kind, the halogenase OocP was found to require the partner protein OocQ for activity. Jointly, OocPQ chlorinate one of the most complex known substrates modified by an Fe(II)/ɑ-KG-dependent halogenase. In this work, we aimed to understand the role of OocQ and its interaction with OocP. Here we report the complex structure of the OocPQ heterodimer and emphasize the significance of the observed interactions for the catalytic function.
Link to the paper in external page "Structure".