Resistance to mesenchymal reprogramming sustains metastatic propagation in breast cancer
A recent "Cell Reports" paper authored by Massimo Saini from the group of Nicola Aceto (IMHS) in collaboration with the DKFZ Heidelberg and the Helmholtz Centre Munich delivers an unexpected paradigm shift in the role of Epithelial-Mesenchymal Transition (EMT) in metastatic breast cancer.
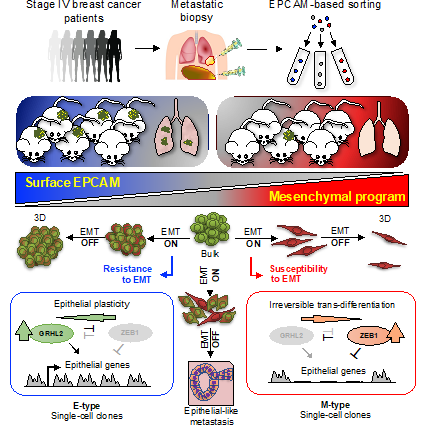
Progression to metastasis is the ultimate cause for most breast cancer deaths. As the disease advances, epithelial cancer cells are thought to acquire mesenchymal traits through a reprogramming event known as the epithelial-mesenchymal transition (EMT). To date, EMT has been historically considered a cornerstone of the metastatic process. However, most studies on EMT have originated from experimental models that suffer from critical limitations, making them difficult to relate to the clinical disease. For this reason, the exact importance of EMT in breast cancer patients was debated.
To address this controversy, researchers at IMHS in a collaborative effort with the DKFZ Heidelberg and the Helmholtz Centre Munich have isolated epithelial and mesenchymal tumor cells directly from metastatic biopsies of breast cancer patients. Then, they tested their pathogenic characteristics at the single-cell level using a variety of advanced techniques, including patient-derived xenografts (PDXs) and 3D organoids.
Surprisingly, EMT was found to reduce metastatic propagation. The researchers discovered that only tumor cells able to oppose resistance to EMT could propagate life-threatening metastasis. By contrast, clones that were susceptible to EMT could not recover their epithelial identity and failed to grow metastases. These observations led the researchers to discover distinct trajectories of cellular reprogramming, explaining why EMT-resistant cells prevail over other breast cancer cells and successfully metastasize. These findings provide important insights favoring the development of more accurate experimental models, enabling a better understanding of how metastasis originates.
Link to the paper in external page "Cell Reports".