Serine protease 35 regulates the fibroblast matrisome in response to hyperosmotic stress
A "Science Advances" paper by the Werner group (IMHS) shows how fibroblasts in culture and in wounded skin respond to hyperosmotic stress by upregulation of serine proteinase 35, which regulates the matrisome and thereby affects cell proliferation.
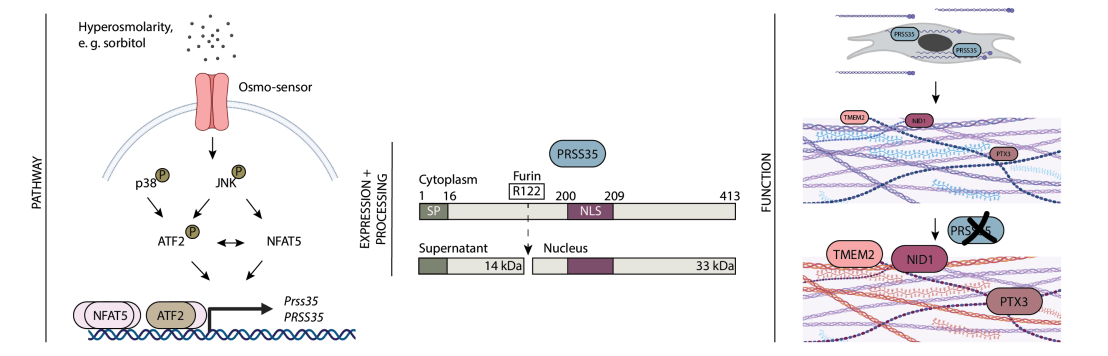
Local osmolarity changes occur transiently after skin wounding. The wound-induced defect in the epidermal barrier leads to enhanced water loss, which augments the osmotic pressure and promotes inflammation. Consistently, moist dressings promote wound healing and reduce scarring, but the underlying mechanisms are poorly understood. Sänger et al. found that exposure of skin fibroblasts to hyperosmotic stress regulates the expression of genes, which help cells to cope with the stress condition. Upregulation of serine protease 35 (PRSS35) was particularly robust and involved stress-regulated kinases and the transcription factors NFAT5 and ATF2. After furin-mediated processing, PRSS35 interacted with collagens and collagen-associated proteins in the secretory pathway of fibroblasts. This is functionally important, because PRSS35 affected the extracellular matrix proteome, which limited cell proliferation. The in vivo relevance of these findings is reflected by the co-expression of PRSS35 and its binding partners in human skin wounds. These results identify PRSS35 as a key regulator of the matrisome under hyperosmotic stress conditions.
Link to the publication in external page "Science Advances".