Structures of connexin-43 gap junction channel and hemichannel in a putative closed state
The recent eLife paper by the group of Volodymyr Korkhov (IMBB, ETHZ & PSI), in collaboration with the Gervasio (UniGe), Bortolozzi (UniPD), Picotti (IMSB, ETHZ) and Zamboni (IMSB, ETHZ) groups describes the structures of connexin-43 gap junction channel and hemichannel in a putative closed state.
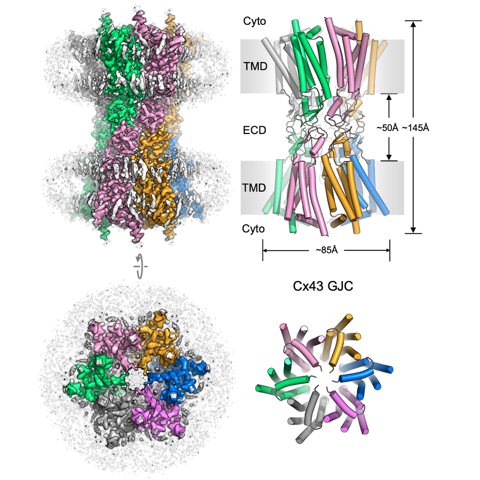
Gap junction channels (GJCs) mediate intercellular communication by connecting two neighbouring cells and enabling direct exchange of ions and small molecules. Cell coupling via connexin-43 (Cx43) GJCs is important in a wide range of cellular processes in health and disease, yet the structural basis of Cx43 function and regulation has not been determined until recently.
The group of Volodymyr Korkhov (PSI & IMBB, ETHZ), in collaboration with the groups of Francesco Gervasio (University of Geneva), Mario Bortolozzi (University of Padua), Paola Picotti (IMSB, ETHZ) and Nicola Zamboni (IMSB, ETHZ) described the structure of a human Cx43 GJC determined by cryo-EM and single particle analysis at 2.26 Å resolution. The pore region of Cx43 GJC features several lipid-like densities per Cx43 monomer, located close to a putative lateral access site at the monomer boundary.
The researchers found a previously undescribed conformation on the cytosolic side of the pore, formed by the N-terminal domain and the transmembrane helix 2 of Cx43 and stabilized by a small molecule. Structures of the Cx43 GJC and hemichannels (HCs) in nanodiscs revealed a similar gate arrangement. The features of the Cx43 GJC and HC cryo-EM maps and the channel properties revealed by molecular dynamics simulations suggested that the captured states of Cx43 may correspond to a novel conformation of the channel, a closed state.
Link to the paper in external page eLife.