Structures of wild-type and selected CMT1X mutant connexin 32 gap junction channels and hemichannels
The recent "Science Advances" paper by the group of Volodymyr Korkhov (IMBB, ETHZ & PSI) in collaboration with the Bortolozzi (UniPD) and Picotti (IMSB) groups describes the structures of Cx32 gap junction channel and hemichannel, providing clues to the molecular defect in CMT1X disease-associated mutant Cx32 hemichannels.
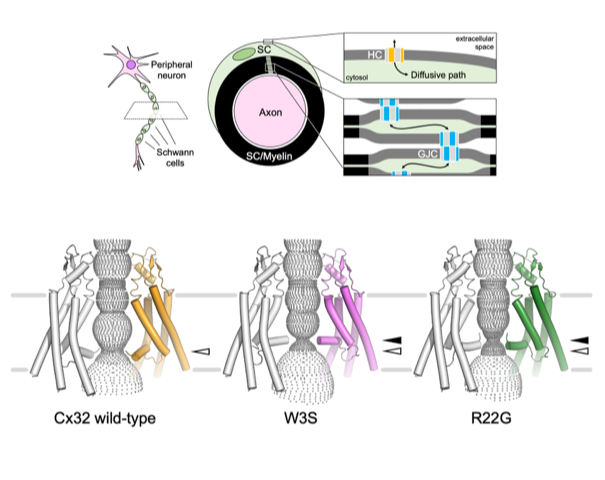
In myelinating Schwann cells, connection between myelin layers is mediated by gap junction channels (GJC) formed by docked connexin 32 (Cx32) hemichannels (HCs). Mutations in Cx32 cause the X-linked Charcot–Marie–Tooth disease (CMT1X), a degenerative neuropathy without a cure. A molecular link between Cx32 dysfunction and CMT1X pathogenesis is still missing.
The group of Volodymyr Korkhov (PSI & IMBB, ETHZ), in collaboration with the groups of Mario Bortolozzi (University of Padua) and Paola Picotti (IMSB, ETHZ) described the high resolution cryo-EM structures of the Cx32 GJC and HC, along with two CMT1X-linked mutants, W3S and R22G. While the structures of wild-type and mutant GJCs are virtually identical, the HCs show a major difference: in the W3S and R22G mutant HCs, the N-terminal gating helix partially occludes the pore, consistent with a diminished HC activity. These results suggest that HC dysfunction may be involved in the pathogenesis of CMT1X.
Link to the paper in external page "Science Advances".