Compacted, yet Flexible: Integrative Structure Determination of Protein RNA Complexes
A "Nature Communications" paper by five ETHZ groups reported an approach for the integrative structure determination of protein RNA complexes containing flexible regions, resulting in several conformations. This approach revealed that PTBP1 acts as a chaperone for a viral IRES RNA.
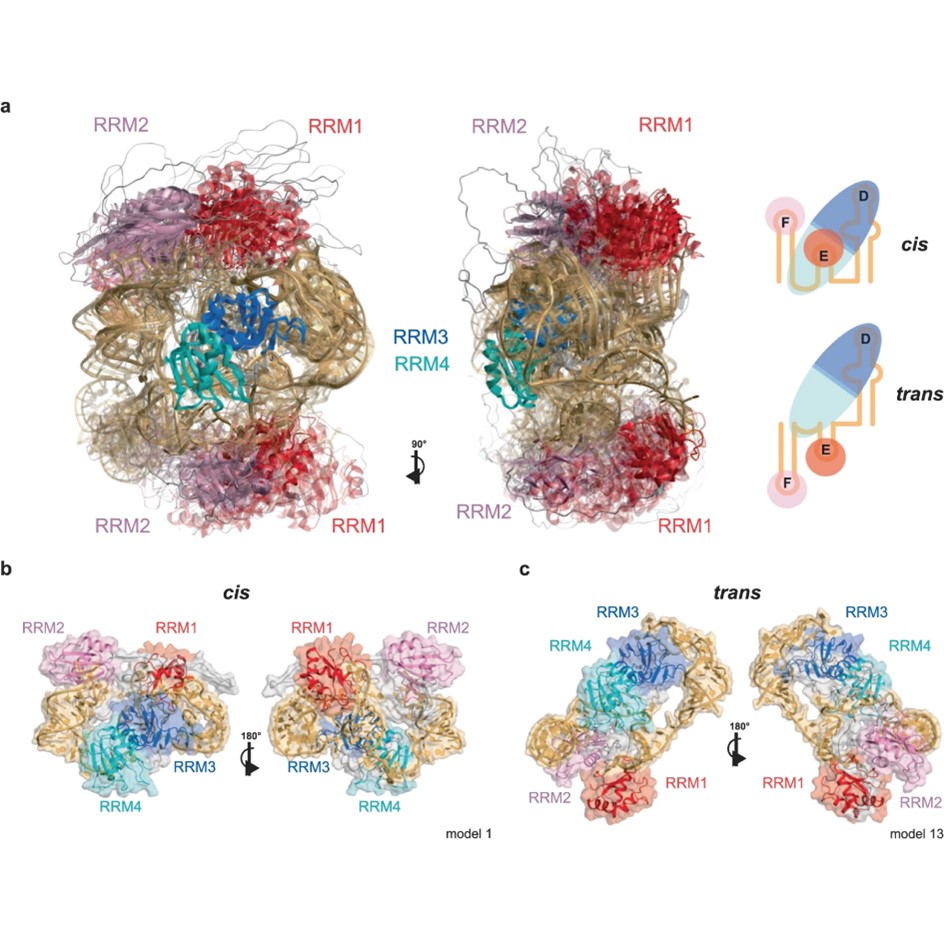
Many RNA binding proteins contain several structured RNA binding domains as well as intrinsically disordered regions (IDRs). While structure determination of the isolated RNA binding domains bound to target RNAs, can be carried out by standard structural methods, this is not the case for full-length proteins containing IDRs bound to longer RNAs, which themselves tend to possess flexibility.
The Allain, Aebersold, Leitner (D-BIOL), Jeschke (D-CHAB) and Mezzenga (D-HEST) groups joined forces to develop an integrative structure determination approach combining data from EPR, NMR, MS, SANS and SAXS. They applied their approach to a protein RNA complex involved in non-canonical, cap-independent translation initiation consisting of the polypyrimidine tract protein 1 (PTBP1) and part of the internal ribosome entry site (IRES) from encephalomyocarditis virus (EMCV) containing three stem-loops.
In their free form, the protein and the RNA display a high degree of flexibility between their respective structured parts, which is greatly reduced upon complex formation, but residual flexibility remains. Due to this, the complex adopts several structural conformations, leaving the stem-loops exposed for the ribosome and additional factors to bind. PTBP1, in this case, acts as an RNA chaperone, guiding the adopted structures of the RNA to be compatible with its function. Based on their findings, the authors think other protein RNA complexes have similar features. Also, their approach should be generally applicable to study protein RNA complexes containing flexible regions adopting several conformations.
Link to the paper in external page "Nature Communications".