In situ analysis of osmolyte mechanisms of proteome thermal stabilisation
A paper from the Picotti group (IMSB) in "Nature Chemical Biology" unravels the mechanisms of widespread protein stabilization by endogenous osmolytes. The work also provides insight into thermal properties of intrinsically disordered proteins and was a collaboration with the Weber-Ban and Arosio groups.
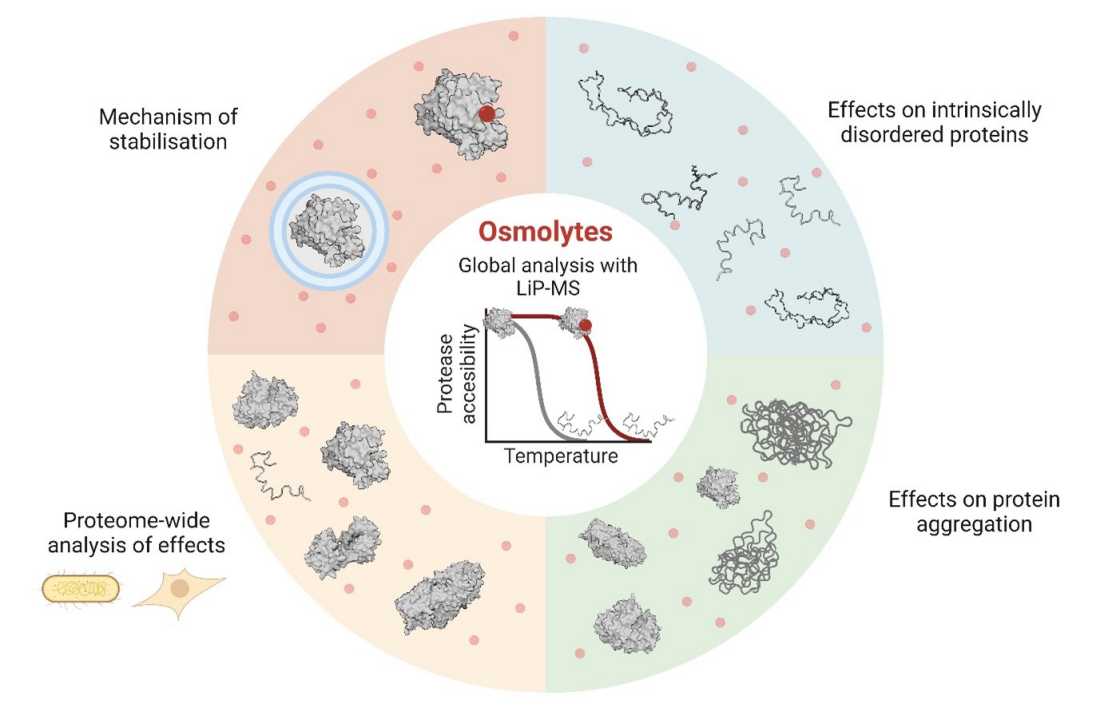
Small molecules called osmolytes are used by many organisms across all kingdoms of life to protect against environmental stressors like heat. It has been known for some time, from studies on a few individual proteins in test tubes, that one of the ways in which this happens is that osmolytes help proteins to stay stable as the temperature goes up. But the exact mechanisms have been controversial and effects of osmolytes on protein thermal stability in the most relevant context - that of the biological cell - was not known.
The Picotti group at IMSB has now shown, for the first time, that osmolytes from six major classes have widespread thermal stabilization effects on proteins in bacterial and human proteomes. Further, since they studied effects on thousands of proteins, they could also ask what mechanisms of stabilization are at play. They found that these vary depending on the protein and the osmolyte, but that a particular mechanism (called preferential exclusion) is most often seen. Also, osmolytes tend to counteract protein aggregation, with some exceptions.
The researchers also generated unprecedented insight into how intrinsically disordered regions (IDRs) of proteins behave within a complex human proteome. IDRs are currently of great interest as they are newly thought to be involved in cellular organization and since many intrinsically disordered proteins are involved in human disease. As expected for a disordered protein region, most predicted IDRs did not show evidence of protein folding in situ. Surprisingly however, about a third of them may fold in situ based on thermal data from this study.
Beyond the biophysical insights into how osmolytes stabilize proteins and how IDRs behave in situ, the study outlines principles for the design of protein stabilizers as well as assays for screening for such stabilizers.
Link to the paper in external page "Nature Chemical Biology".