02
In situ analysis of osmolyte mechanisms of proteome thermal stabilisation
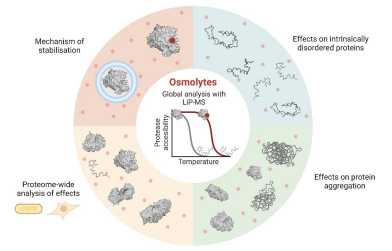
A paper from the Picotti group (IMSB) in "Nature Chemical Biology" unravels the mechanisms of widespread protein stabilization by endogenous osmolytes. The work also provides insight into thermal properties of intrinsically disordered proteins and was a collaboration with the Weber-Ban and Arosio groups.
A Lysyl Oxidase-Responsive Collagen Peptide Illuminates Collagen Remodeling in Wound Healing
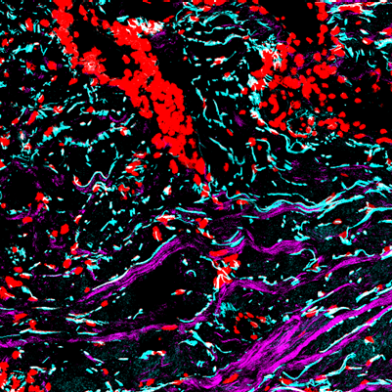
A recent publication in “Matrix Biology”, which resulted from a collaboration between D-CHAB (Wennemers group) and D-BIOL (Werner group) within the frame of the SKINTEGRITY.CH project, demonstrates that an innovative chemical sensor offers visualization of collagen remodeling during tissue repair.
Regulatory sites of CaM-sensitive adenylyl cyclase AC8 revealed by cryo-EM and structural proteomics
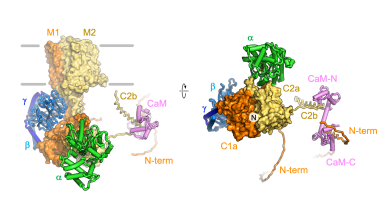
The recent EMBO Reports paper by the group of Volodymyr Korkhov (IMBB, ETHZ & PSI) in collaboration with the groups of Paola Picotti (IMSB, ETHZ) and Alexander Leitner (IMSB, ETHZ) describes the integrative structural biology analysis of adenylyl cyclase AC8 and its complexes with regulatory proteins (Gαs, Gβγ and calmodulin).
Dimerization of a 5 kDa domain defines the architecture of a 5 MDa mega-enzyme complex
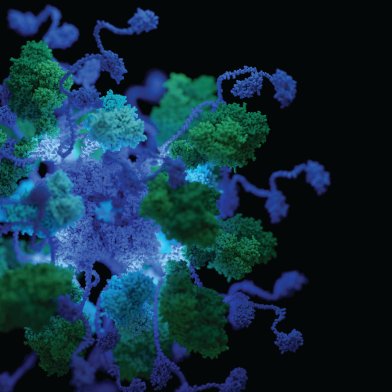
In a recent paper in Science Advances, the Glockshuber group (IMBB) solves a 60-year-old conundrum and shows how dimerization of a 5 kDa domain determines the architecture and stoichiometry of the 5 MDa pyruvate dehydrogenase multienzyme complex from Gammaproteobacteria.
ETH GreenLabs: first labs certified for sustainability

Laboratory operations are associated with a considerable environmental impact. It was the goal of a pilot project at ETH Zurich and the University of Zurich to explore the potential for resource-saving laboratory processes. On Wednesday, 31st of January, the first certification ceremony of the pilot took place, and several D-BIOL labs were awarded certificates.