FUS Droplets: Growing a Shell with Age
In a "Nature Chemical Biology" paper, the Allain lab (IBC) and collaborators including the Michaels group (IBC) mechanistically describe how droplets of the protein fused in sarcoma (FUS) mature. They grow over time a hard shell, which plays a critical role in droplet function.
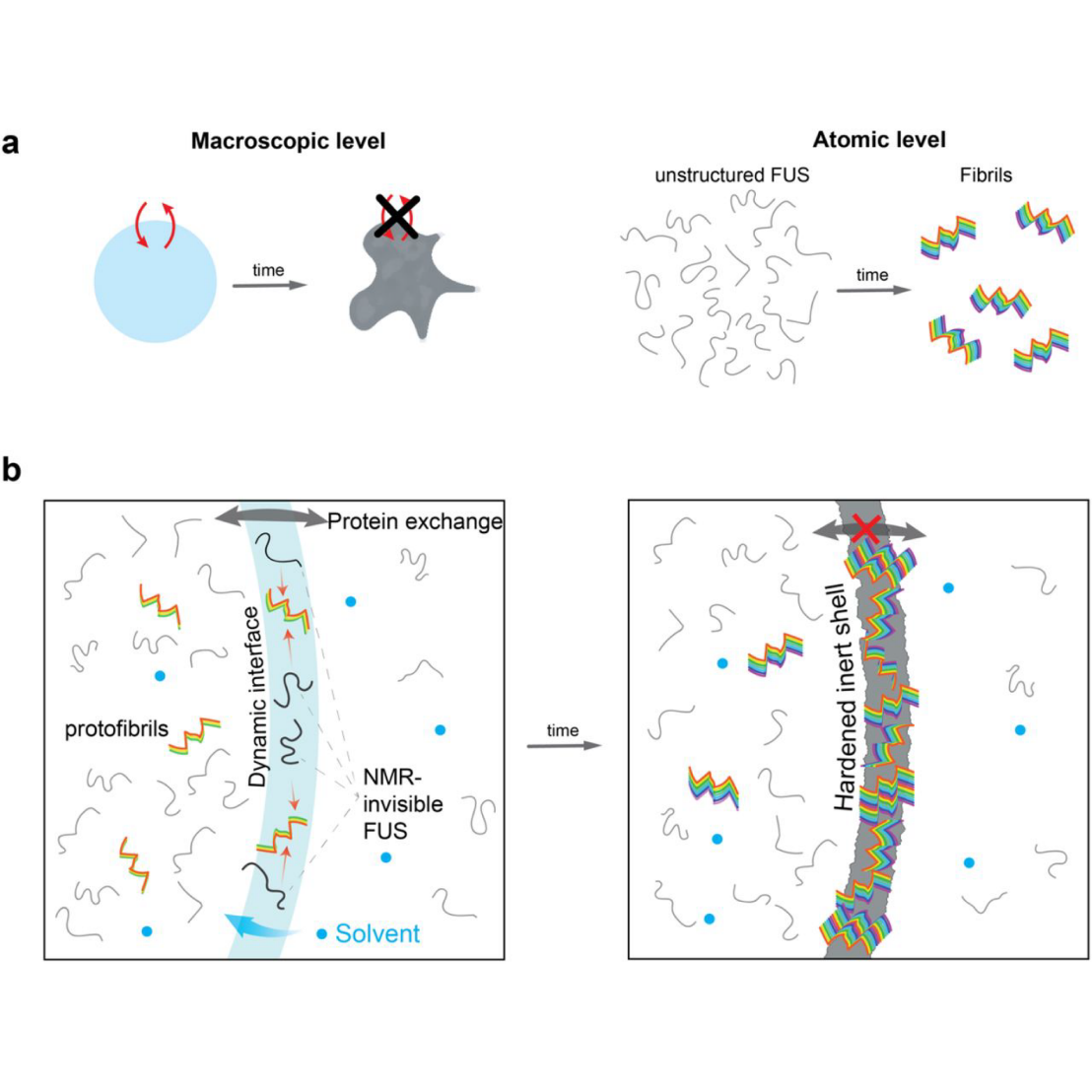
Mutations in the FUS protein lead to genetic forms of amyotrophic lateral sclerosis (ALS) with irreversible FUS aggregates which could arise from FUS droplets. These droplets form without RNA and rigidify over time. This process is faster for droplets made up of FUS containing disease-related mutations.
The researchers studied the maturation of the FUS droplets embedded in an agarose matrix. This biphasic system contains FUS in solution and in droplets of similar size as in cells, leading to a larger surface-to-volume ratio than in the often-studied monophasic FUS droplet systems. Using solution and solid state NMR as well as Raman spectroscopy and microaspiration, they followed the maturation of FUS droplets for up to months. They found that droplets in the biphasic agarose matrix system grow a rigid shell containing beta-sheet fibrillar structures. Intriguingly, in the presence of RNA this shell does not form.
Their data allowed the researchers to develop a mechanism for the liquid-to-solid-transition of FUS and find that the droplet surface is an additional subcellular region whose properties may be essential for droplet function. Their findings also reconcile the opposing views that Beta sheet structures or weak unspecific interactions hold together droplets.
Link to the paper in external page "Nature Chemical Biology".