Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP
A recent article in the journal “Nature Communications” by the Locher group (IMBB) in collaboration with the Kossiakoff group (The University of Chicago), the Urban group (Heidelberg University), the Glebe group (Justus Liebig University Giessen), and the Geyer group (Justus Liebig University Giessen) describes the structural basis of mimicked HBV/HDV viral peptide drug interaction with its receptor NTCP.
HBV, an enveloped DNA virus, causes acute and chronic infection of the liver and the chronic form, significantly contributes to the overall burden of liver-related diseases, such as cirrhosis and hepatocellular carcinoma (HCC). Additionally, the hepatitis D virus (HDV), an enveloped RNA satellite virus that uses HBV surface proteins in HBV/HDV co-infection, enhances the severity of liver disease. NTCP (SLC10A1), involved in the enterohepatic circulation of bile salts, was identified as a receptor for HBV/HDV. Previous studies have shown that the viral interaction with NTCP is mediated by the N-terminal myristoylated preS1 domain of the large surface protein of HBV/HDV. However, the exact mechanism of NTCP-mediated virus recognition remained elusive. It was recognized that strategies aimed at inhibiting or disrupting binding of preS1 to NTCP hold promise in preventing HBV/HDV infection. In 2023, the commercially available drug bulevirtide (BLV, also known as Hepcludex®), received market approval for the treatment of chronic HDV infection in Europe. BLV exhibits a remarkably high inhibition against HBV and HDV, and BLV has also been demonstrated to inhibit the NTCP-mediated uptake of bile salts.
The group of Prof. Kaspar Locher (IMBB, ETHZ), with collaborators, reported a cryo-EM structures of viral peptide bulevirtide-bound human NTCP at a resolution 3.4 Å. The studies provide insight into the mechanism of BLV inhibition and into the interaction between NTCP and the viral preS1 peptide. Coupled with functional analysis, the study advances the molecular understanding of how BLV blocks HBV/HDV infection, which hold promise for developing additional therapeutic interventions against HBV/HDV, preventing viral entry into hepatocytes, and thus reducing HBV/HDV-related liver damage.
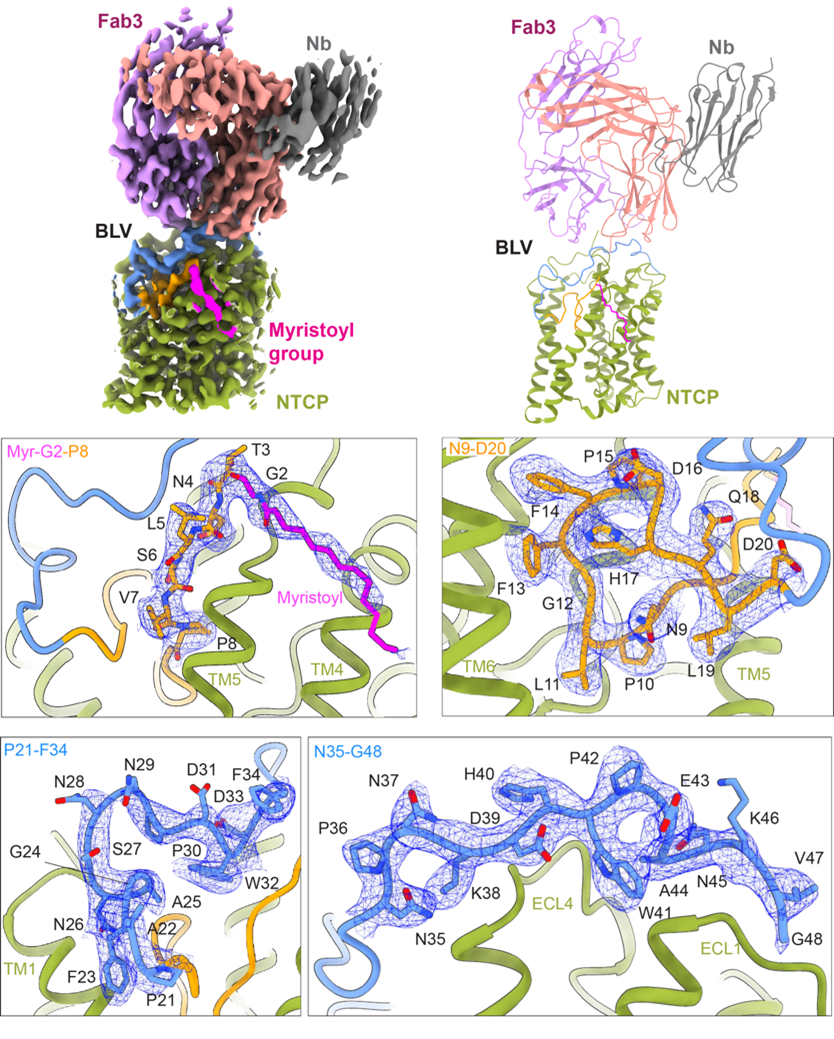
Liu H., et al., showed that the nanodisc-reconstituted NTCP can be captured by the patient-derived HBV subviral particles (SVPs), demonstrating binding of NTCP to the SVPs. TC transport by NTCP was fully inhibited at high concentrations of BLV. To gain structural insight into how BLV interacts with NTCP, we generated an antigen-binding antibody fragment (Fab) specific to the NTCP-BLV complex. We obtained a 3.4 Å EM density map revealing excellent density for NTCP, bound BLV, and Fab3. The EM map revealed well-resolved density for BLV located both in the tunnel and on the surface of NTCP, allowing us to build all 47 amino acids and the myristoyl moiety of the BLV peptide. The myristoyl group of BLV interacts with the surface of TM4 and TM5 of NTCP and is exposed to lipids bilayer. Starting with Gly2, the first 19 residues of BLV adopt a globular shape that forms a plug wedged inside the translocation tunnel and reaching the middle of the membrane bilayer. The string domain of BLV (Pro21-Gly48) covers the surface of the plug and crosses the extracellular surface of NTCP. In conclusion, our study reveals the molecular basis of viral preS1 interactions with its receptor NTCP and the inhibition of this interaction by the commercial drug bulevirtide. Our findings allow us to rationalize HBV/HDV specificity for human NTCP and explain why the S267F mutation in humans presents a compromised bile salt transport with simultaneous resistance to HBV/HDV. These might be amenable for the design of smaller drugs (peptidomimetics) that improve the pharmacology of BLV to prevent HBV and HDV binding and infection.
Link to the paper in external page "Nature Communications".