Transport of CLCA2 to the nucleus by extracellular vesicles controls keratinocyte survival and migration
A recent "Journal of Extracellular Vesicles" paper by the Werner group (IMHS) identified an unexpected nuclear transport mechanism of the stress-regulated transmembrane protein CLCA2 by extracellular vesicles (EVs). The presence of CLCA2 in the nucleus is required for the regulation of key cellular functions in skin keratinocytes.
Chronic inflammatory skin diseases, such as atopic dermatitis and psoriasis, affect millions of people worldwide and strongly impact their quality of life. A key pathogenic feature of these disorders is the disruption of the epidermal barrier through defects in keratinocyte adhesion.
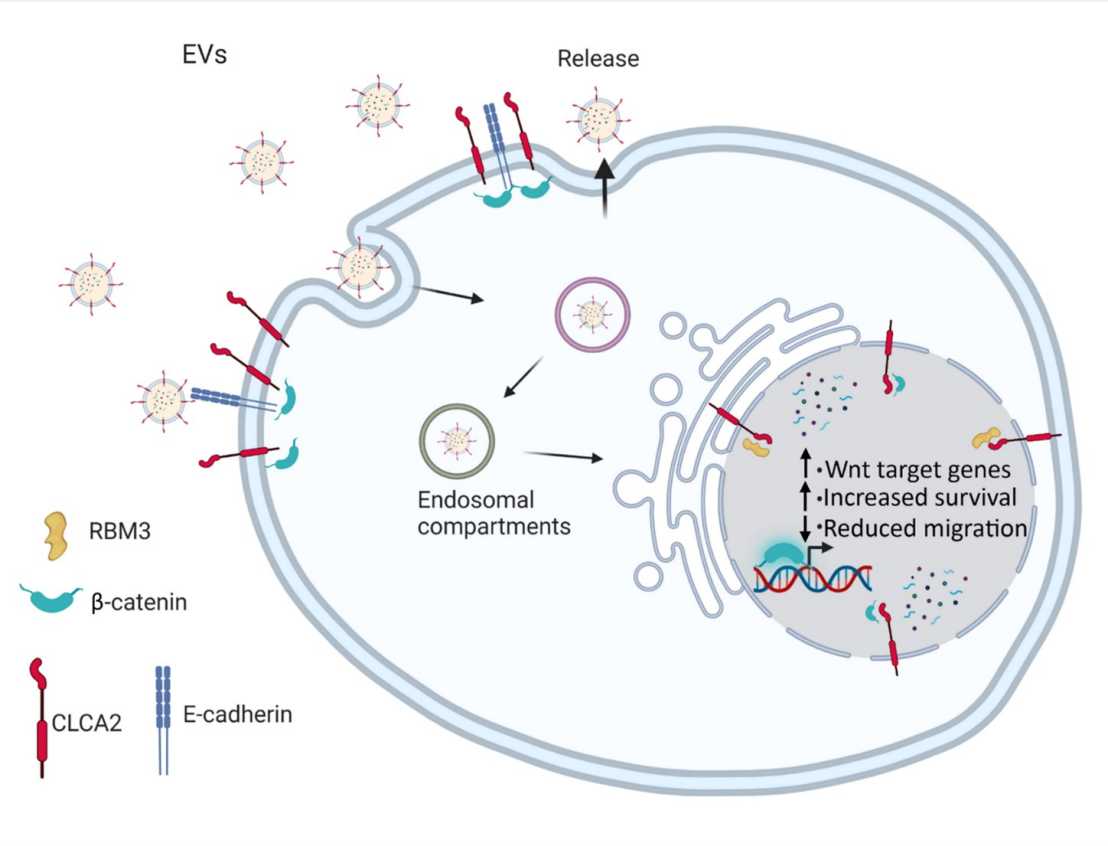
Previous research of the Werner group identified the transmembrane protein chloride channel accessory 2 (CLCA2) as a stress-regulated protein in keratinocytes, which promotes keratinocyte adhesion and protects them from cell death in response to hyperosmotic stress, which occurs in a dry environment. Their new study shows that CLCA2 also localizes to the nucleus, to which it is transported via extracellular vesicles (EVs). In this compartment, CLCA2 binds to β-catenin and RNA binding motif protein 2 (RBM3), thereby regulating the expression of Wnt target genes. Nuclear CLCA2 is also important for the promotion of keratinocyte survival under hyperosmotic stress conditions and the suppression of keratinocyte migration.
The interaction between CLCA2 and RBM3 is of likely relevance in vivo, because both proteins are co-localized in the human epidermis. Importantly, CLCA2 is overexpressed in the epidermis of atopic dermatitis patients, where it likely protects keratinocytes from stress caused by a weakened skin barrier. These results identify an unexpected nuclear function of CLCA2 in keratinocytes under homeostatic and stress conditions and suggest a role of EVs and their nuclear transport in the control of key cellular activities.
Link to the paper in the external page "Journal of Extracellular Vesicles".