Structural basis of the Meinwald rearrangement catalysed by styrene oxide isomerase
The recent Nature Chemistry paper by the group of Volodymyr Korkhov (IMBB, ETHZ & PSI) in collaboration with Xiaodan Li and Richard Kammerer (PSI) and other collaborators, describes that structure of a bacterial styrene oxide isomerase (SOI), a membrane enzyme that catalyzes the Meinwald reaction, isomerizing an epoxide to a carbonyl compound.
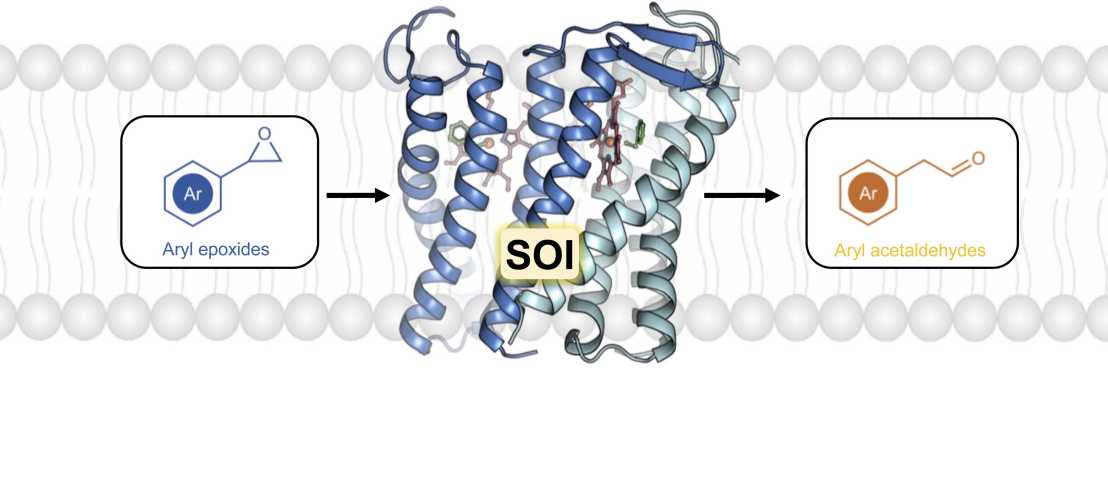
SOI is an integral membrane protein that catalyses the Meinwald rearrangement, a Lewis-acid-catalysed isomerization of an epoxide to a carbonyl compound. The enzyme has been used in single and cascade reactions. However, the structural basis of its reaction mechanism has remained elusive. In a study led by Xiaodan Li and Richard Kammerer (PSI) in collaboration with the Korkhov group (Basavraj Khanppnavar, PSI & Institute of Molecular Biology and Biophysics, ETH Zurich), as well as the groups of Dirk Tischler (Ruhr University Bochum), Peter-Leon Hagedoorn (Delft University of Technology) and Zhi Li (National University of Singapore), the authors determined the cryo-electron microscopy (cryo-EM) structures of Pseudomonas sp. VLB120 SOI bound to a single-domain antibody with and without the competitive inhibitor benzylamine, and elucidated the catalytic mechanism using electron paramagnetic resonance spectroscopy, functional assays, biophysical methods and docking experiments.
They found that the enzyme has ferric haem b bound at the subunit interface of the trimeric enzyme through H58, where Fe(III) acts as the Lewis acid by binding to the epoxide oxygen. Y103 and N64 and a hydrophobic pocket binding the oxygen of the epoxide and the aryl group, respectively, position substrates in a manner that explains the high regio-selectivity and stereo-specificity of SOI. These findings can support extending the range of epoxide substrates and be used to potentially repurpose SOI for the catalysis of new-to-nature Fe-based chemical reactions.
Link to the paper in external page "Nature Chemistry".