Architecture and composition of the human pyruvate dehydrogenase complex elucidated
In a recent "Science Advances" paper, the Glockshuber group (IMBB), together with the cryo-EM hub, the Zenobi group (D-CHAB) and Alexander Leitner (IMSB) solved the long-standing conundrum of the precise subunit composition and core architecture of the mammalian pyruvate dehydrogenase complex.
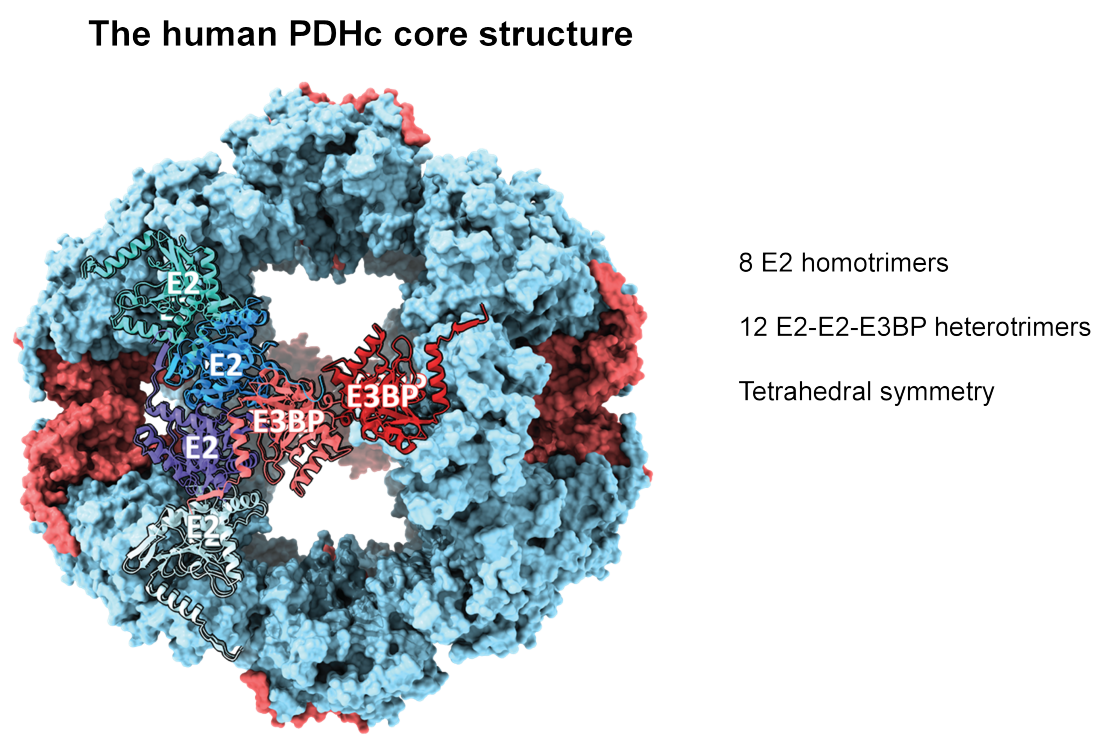
The pyruvate dehydrogenase complex (PDHc) is an essential mega-enzyme in all aerobic organisms and links glycolysis with the citric acid cycle by catalyzing the oxidative decarboxylation of pyruvate to acetyl-CoA. PDHc is composed of multiple copies of three enzymes: pyruvate dehydrogenase (E1), dihydrolipoamide acetyl transferase (E2) and dihydrolipoamide dehydrogenase (E3).
Previous studies had shown that the core of mammalian PDHcs is a pseudo-icosahedral hetero-oligomer of the subunits E2 and the E3 binding protein (E3BP), but the exact number of E2 and E3BP copies remained a long-standing mystery. In the present study, Zdanowicz et al. showed that the core of human and porcine PDHc contains 48 copies of E2 and 12 copies of E3BP. The 12 E3BP subunits are arranged tetrahedrally within the core particle formed from 8 E2 homotrimers and 12 E2-E2-E3BP heterotrimers. Zdanowicz et al. also showed that up to 48 heterotetramers of the peripheral subunit E1 can bind to the 48 E2 subunits of the core, and up to 12 E3 homodimers bind to the 12 E2BP copies of the core. Mammalian PDHc, fully saturated with E1 and E3, thus has a mass of 12.0 MDa and is about 3 times larger than the mammalian ribosome.
The even distribution of the peripheral subunits around the core guarantees efficient transfer of catalytic intermediates between the active sites of E1, E2 and E3 and maximum PDHc activity. The E1 heterotetramer:E2 monomer:E3 homodimer ratio of 48:48:12 allowed the determination of the exact catalytic parameters of human PDHc that now can be used for calculating metabolic fluxes in human cells.
Link to the paper in external page "Science Advances".