Structural basis of connexin-36 gap junction channel inhibition
The new study published in Cell Discovery by the group of Volodymyr Korkhov (IMBB, ETHZ & PSI) jointly with group of Francesco Gervasio (University of Geneva) and other collaborators describes the mode of gap junction channel inhibition by several small molecule drugs.
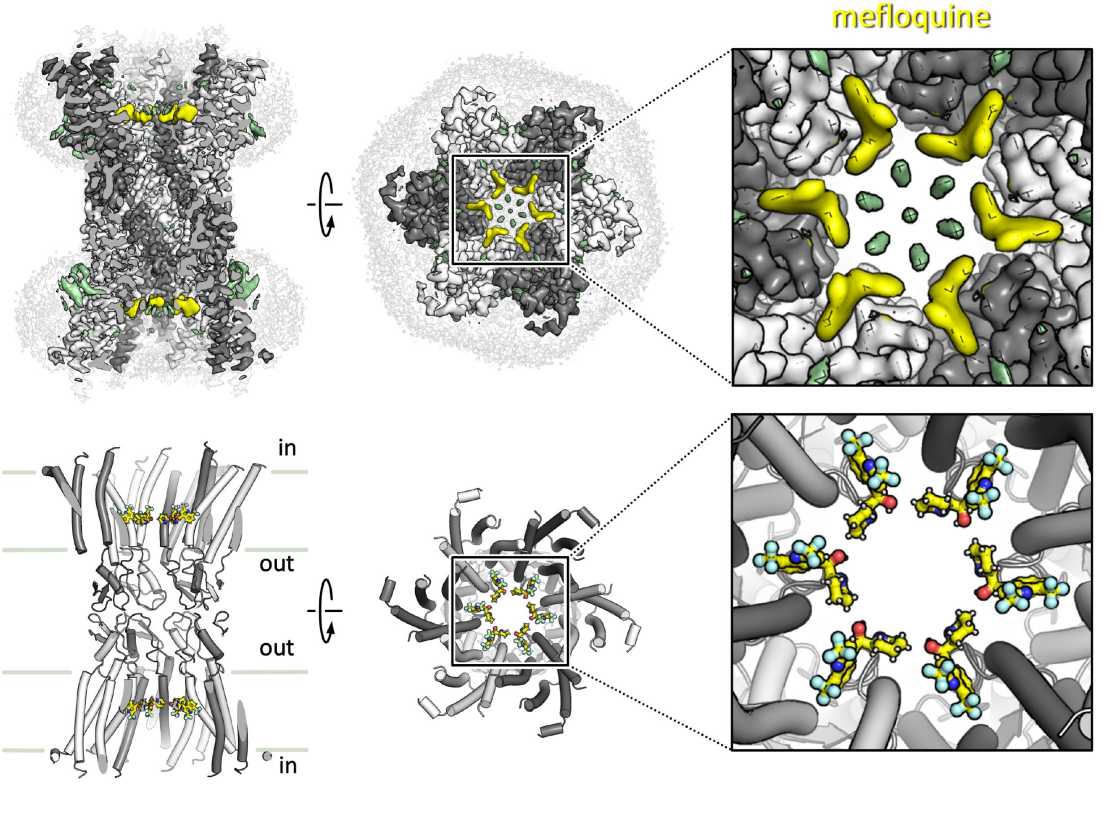
Connexin gap junction channels and hemichannels play important roles in intercellular communication and signaling. Some of connexin isoforms are associated with diseases, including hereditary neuropathies, heart disease and cancer. Although small molecule inhibitors of connexins show promise as therapeutic agents, the molecular mechanisms of connexin channel inhibition are unknown. The Korkhov group (Institute of Molecular Biology and Biophysics, ETH Zurich & Paul Scherrer Institute), in collaboration with the groups of Francesco Gervasio (University of Geneva) and Paola Picotti (Institute of Molecular Systems Biology, ETH Zurich), determined the cryo-EM structure of connexin-36 (Cx36) bound to an anti-malarial drug mefloquine at 2.1 Å resolution.
The structure showed that six drug binding sites partially occlude the pore of each connexon forming the channel. Each drug molecule in the ring makes contacts with residues of the pore-lining pocket and with the neighbouring mefloquine molecules, partially occluding the pore and modifying the pore electrostatics, ultimately reducing solute translocation through the channel. Structures of Cx36 in the presence of quinine and quinidine showed a similar mode of drug binding. Molecular dynamics simulations of Cx36 bound to mefloquine revealed that drug binding affects the kinetics of ion passage through the pore. This previously undescribed mode of connexin channel inhibition points to new opportunities for designing subtype-specific connexin inhibitors.
Link to the paper in external page "Cell Discovery".