Partial closure of the gamma-tubulin ring complex by CDK5RAP2 activates microtubule nucleation
A recent “Developmental Cell” paper by the Wieczorek group (IMBB) reports a novel structure of the mammalian gamma-tubulin ring complex and demonstrates how the centrosomal protein CDK5RAP2 stimulates its microtubule-nucleating activity at sites of microtubule assembly.
Microtubules are cytoskeletal polymers self-assembled from thousands of protein subunits called tubulin. The formation of new microtubules, a process also called “nucleation”, is carefully regulated in cells. Defective microtubule nucleation is linked to developmental disorders and defects in cell division.
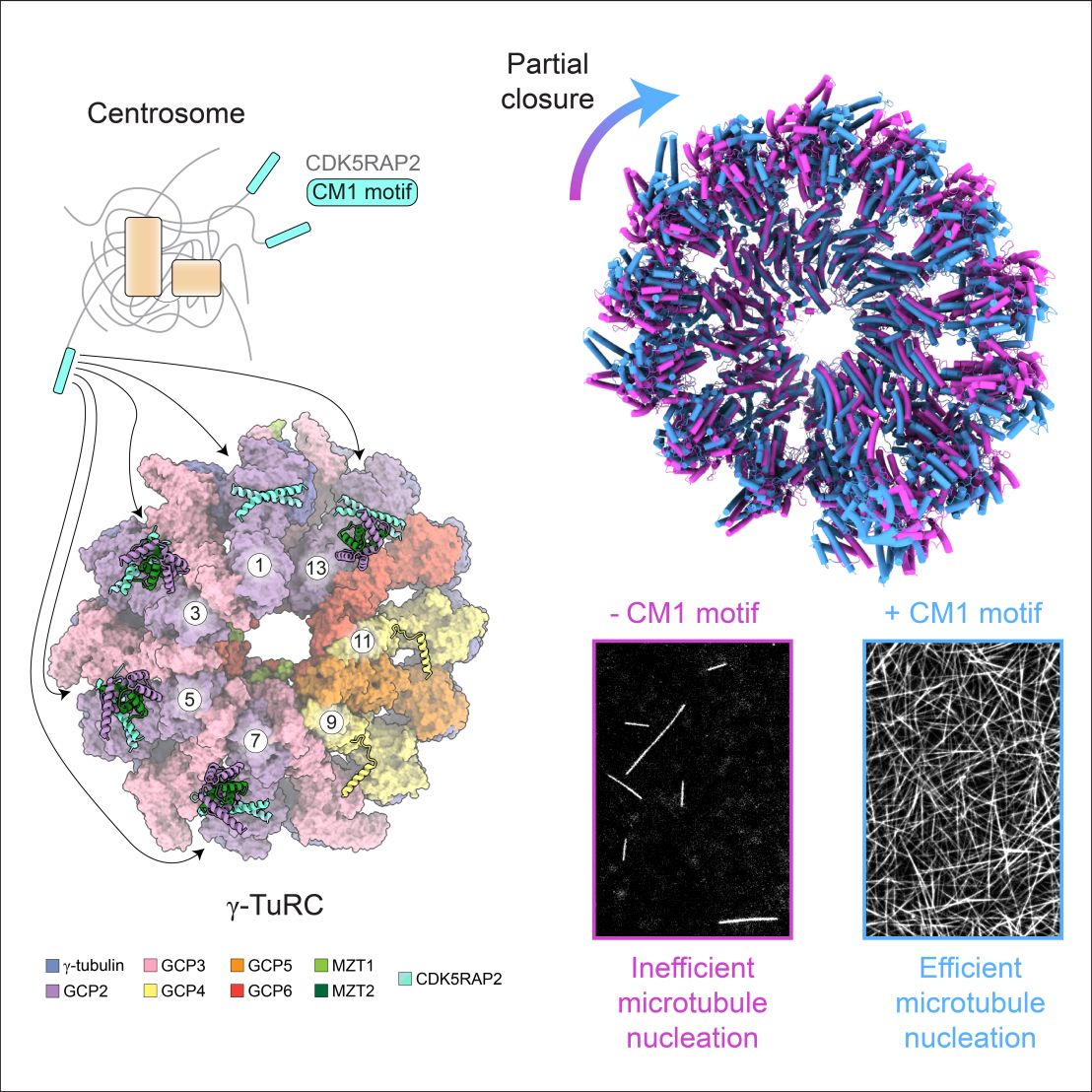
Correctly nucleating a microtubule at the right time and place is difficult. Cells overcome this challenge by making a template for the microtubule called the gamma-tubulin ring complex (gamma-TuRC). Previous structural work showed that the gamma-TuRC is in an open conformation that mimics, but does not match, the microtubule. This structural mismatch may be the reason why the gamma-TuRC is a poor microtubule nucleator in vitro. gamma-TuRC-associated proteins, including the centrosomal protein CDK5RAP2, have been proposed to activate the gamma-TuRC by correcting this mismatch, but the molecular details are not clear.
Researchers at IMBB have discovered that CDK5RAP2 induces a structural change in the gamma-TuRC that paritally closes the complex and brings its conformation much closer to the architecture of a microtubule. By tracking single microtubule nucleation events, they show that CDK5RAP2 activates the nucleating activity of the gamma-TuRC and dissect the parts of the complex required for this effect.
The study provides a key starting point for exploring how cells use CDK5RAP2 and other, as yet unexplored adaptor proteins, to control where and when microtubule nucleation occurs.
Link to the paper in external page "Developmental Cell".