Ribosomal peptides with polycyclic isoprenoid moieties
A recent “Chem” paper by the Piel group (IMB) in collaboration with the Chekan (UNC Greensboro) and Gugger groups (Institut Pasteur) reports a new and unusually complex steroid-like post-translational modification.
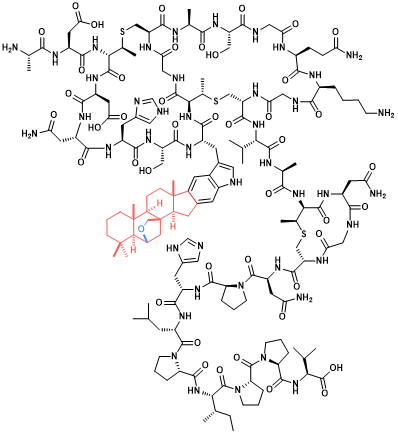
Isoprenoid post-translational modifications of proteins and peptides serve fundamental biological functions and are of therapeutic interest. While C15 (farnesyl) and C20 (geranylgeranyl) moieties are prevalent among proteins, known ribosomal peptide prenylations involve shorter-chain units not exceeding farnesyl in size. Cyclized terpene moieties have not been reported from either biomolecule class.
In this paper, the researchers used targeted genome mining and heterologous pathway reconstitution to identify peptides with elaborate cyclized C20 modifications. The peptide-modifying enzymes commonly feature fused prenyltransferase-terpene cyclase architectures. Two bifunctional maturases with distinct prenyltransferase folds were characterized, and the terminal product of a cyanobacterial pathway identified as an exceptionally complex peptide containing a heptacyclic amino acid with a steroid-like modification. Bioassays suggest anti-cyanobacterial activity with the modification being crucial for activity. Genome data predict similar cyclic isoprenoid units for various ribosomal peptide families and thus broader natural and biotechnological compatibility of the pseudosteroid modification system.
Link to the paper in external page "Chem".