An exploration into the depths of T helper cell heterogeneity and functional plasticity
A recent report in «Immunity» by the Kopf group (IMHS) reveals the plethora of stable T helper (Th) cell sublineages and uncovers unexpected developmental relationships between Th cell subsets.
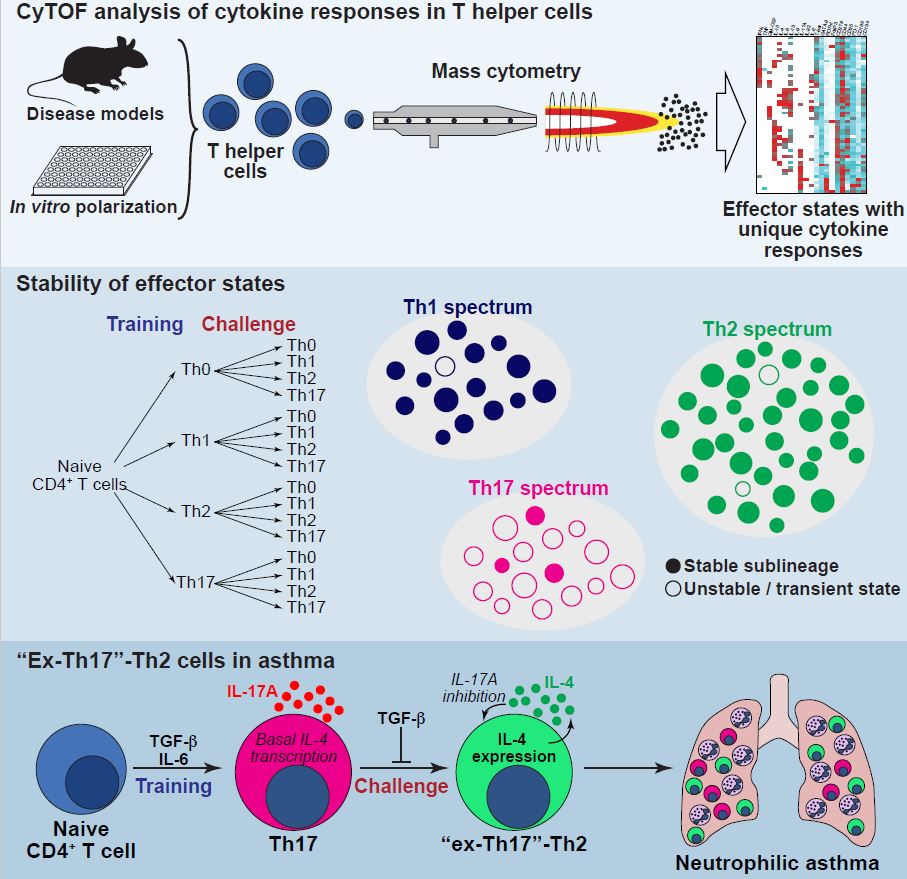
T helper (Th) cells are the orchestrators of adaptive immune responses. By secreting an army of cytokines, they coordinate the function of all other immune cells. Based on the expression of signature cytokines and transcription factors, distinct prototypical Th subsets such as Th1, Th2, and Th17 with specific functions have been defined. Nonetheless, Th cell heterogeneity is believed to extend beyond the known subsets. Characterization of their full diversity holds the key to a better understanding of adaptive immunity, but has thus far been hindered by technical limitations.
In this study, immunologists from the Kopf group (IMHS) teamed up with the Bodenmiller lab at the Department of Quantitative Biomedicine (UZH) to investigate the diversity of cytokine responses generated by effector Th cells by employing mass cytometry (aka CyTOF), a recently developed high-dimensional single cell analysis technology.
They revealed that prototypical Th cell subsets consist of a mesmerizingly heterogeneous cloud of Th cell effector states with distinct cytokine expression patterns among the conventional lineages of Th cells. In follow-up experiments to determine the stability and plasticity of these effector states, they found that most of them were not transient, unstable states of differentiating cells on their way to their final stage, but rather stable sublineages, whose cytokine footprint was stably inherited. This was however not always the case. The authors found that de novo generated Th17 cells were unstably committed to their lineage and were predisposed to acquire a Th2-like cell phenotype after challenge. Using an in vivo system to track the fate of Th17 cells, they found that ‘‘ex-Th17’’-Th2 cells contributed large amounts of pathogenic cytokines in neutrophilic allergic airway inflammation, a severe form of asthma.
This work has repercussions on our understanding of Th cell population behavior, suggesting that functional plasticity of Th cells arises from dynamic changes in the cloud of heterogeneous sublineages and defining the unexpected developmental relationship of Th17 and Th2 cells.
Link to the publication in external pageImmunity.