A sacrificing subpopulation of bacteria releases virulence factors for infection
A recent "Nature Microbiology" paper by the Pilhofer Lab (IMBB) unveils the assembly and release of Tc toxins from Yersinia entomophaga. Expressed in only a subpopulation alongside other virulence factors, these toxins are then released via cell lysis mediated by a phage-like lysis cassette.
Tripartite toxin complexes (Tc toxins) are virulence factors of bacterial pathogens. These megadalton complexes employ a syringe-like mechanism to translocate and disrupt cellular processes, leading to cell death. Although the intoxication mechanism of Tc toxins is understood well, the site of assembly and the release of these large complexes across the bacterial cell envelope into the extracellular space remained elusive until now.
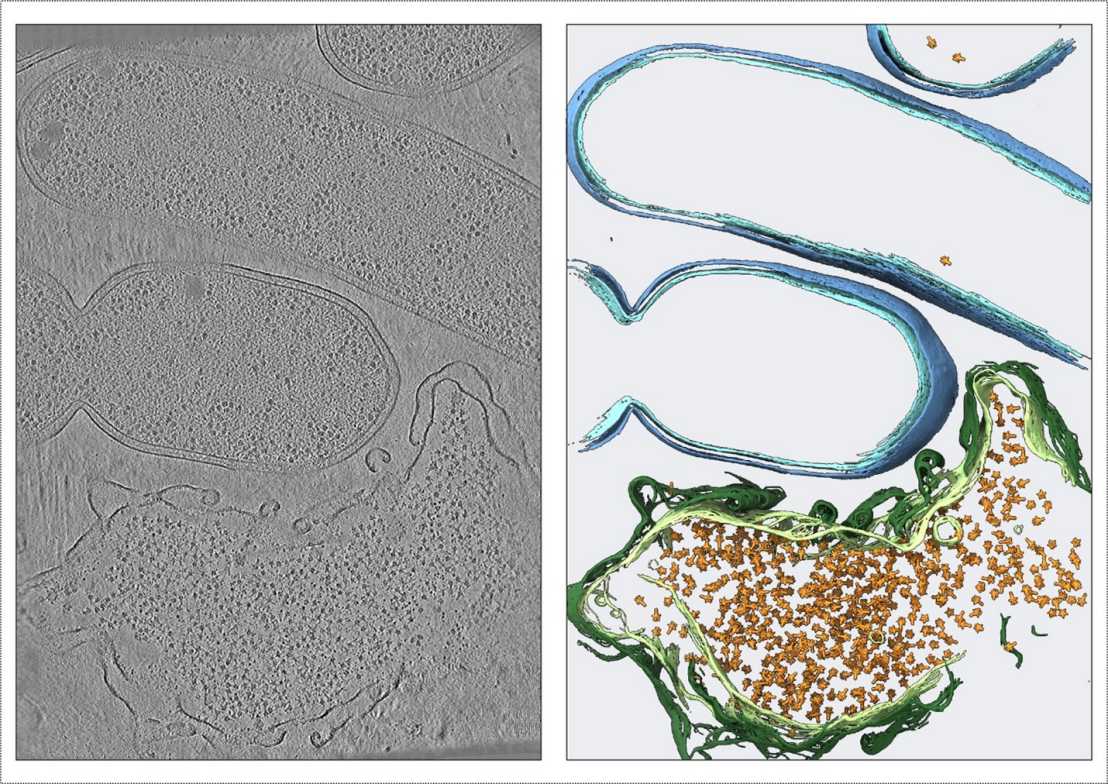
In their recent study, researchers have uncovered the intricate mechanism behind the assembly and release of Tc toxins (YenTc) from the insect pathogen Yersinia entomophaga.
Utilizing an integrative multiscale imaging approach including correlative cryo-light and electron tomography, time-lapse light microscopy and proteomics, the scientists found that YenTc toxin components are expressed only in a subpopulation of cells, which are 'primed' also with other potential virulence factors, including filaments of the protease M66/StcE. The key to the release of YenTc and other virulence factors lies in a bacteriophage-like lysis cassette, which, rather than causing immediate cell lysis, generates intermediate 'ghost' cells. These ghost cells serve as assembly compartments, densely packed with assembled YenTc holotoxins (Figure 1).
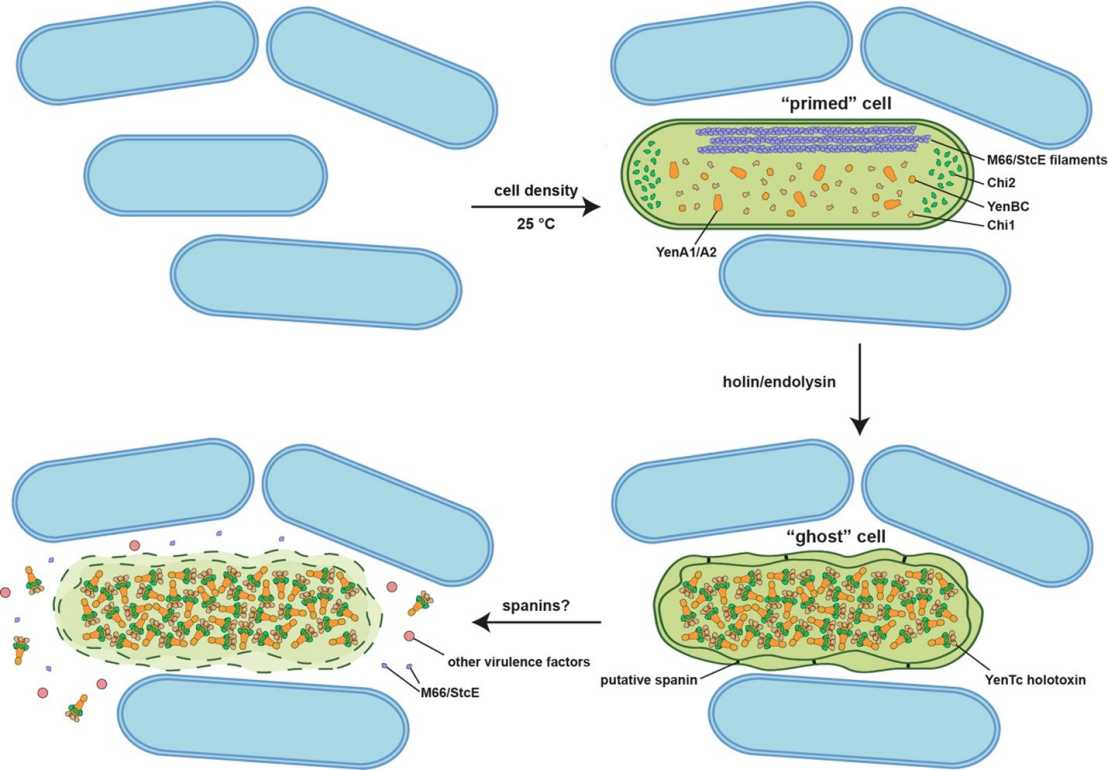
The researchers hypothesize that this stepwise mechanism evolved to minimize the number of cells needed to be killed, a strategy that could confer a fitness advantage to the bacterial community (Figure 2). The conservation of a phage-like lysis cassettes in other Tc toxin-positive species, hints at a universal mechanism for Tc toxin release and may apply to the release of other virulence factors, addressing the broader challenge of bacteria having to release large macromolecular complexes into their environment to mediate cell-cell interactions.
Link to the paper in external page "Nature Microbiology".