Inhibition mechanism of human oligosaccharyltransferase OST-A by the drug NGI-1
A recent article in the journal "Cell" by the Locher group (IMBB) in collaboration with Kaelin (Dana-Farber Cancer Institute) and Contessa (Yale School of Medicine) groups revealed how the human OST-A enzyme is inhibited by the drug NGI-1.
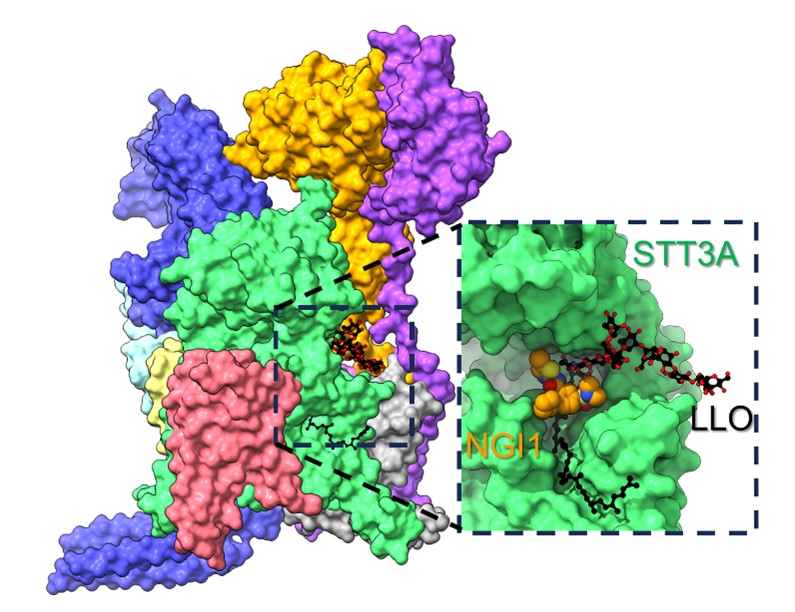
Human oligosaccharyltransferase (OST) is a multimeric membrane protein that catalyzes the transfer of a pre-assembled glycan from a lipid-linked oligosaccharide (LLO) to acceptor proteins in the lumen of the endoplasmic reticulum. The attached glycans (N-glycans) play essential roles in diverse cellular processes, including protein folding, trafficking, and targeting. Recent advances in the discovery of small molecule inhibitors of human OST enzymes, such as NGI1, revealed their potential as an antiviral drug and adjuvant to chemotherapy for cancer treatment.
The Toll-like receptor 4 (TLR4) binds bacterial lipopolysaccharide (LPS) and initiates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signal transduction pathway, which regulates many physiologic processes in mammals. In this study, Lampson BL and Ramírez AS, et al. revealed that TLR4 is a specific substrate of OST-A, and inhibition of OST-A by NGI1 impairs NF-κB signaling.
By combining genetic and structural approaches, they revealed that NGI1 binds directly to STT3A, the catalytic subunit of OST-A. Specifically, NGI1 binds to a state of the enzyme where the donor LLO is already bound, altering its binding pose and trapping an inactive OST-LLO-NGI1 complex. These results rationalize the molecular basis of OST inhibition by NGI-1, opening new avenues for designing specific inhibitors with pharmacotherapeutic potential.
Link to the paper in external page "Cell".