Reversible amyloids of pyruvate kinase couple metabolism and stress granule disassembly
A recent "Nature Cell Biology" paper by the Peter group (IBC) in collaboration with the Sauer and Picotti groups (IMSB) elucidates a molecular mechanism that is critical for cell survival to stress, revealing how proteins can directly sense the cellular energy levels and reversibly aggregate.
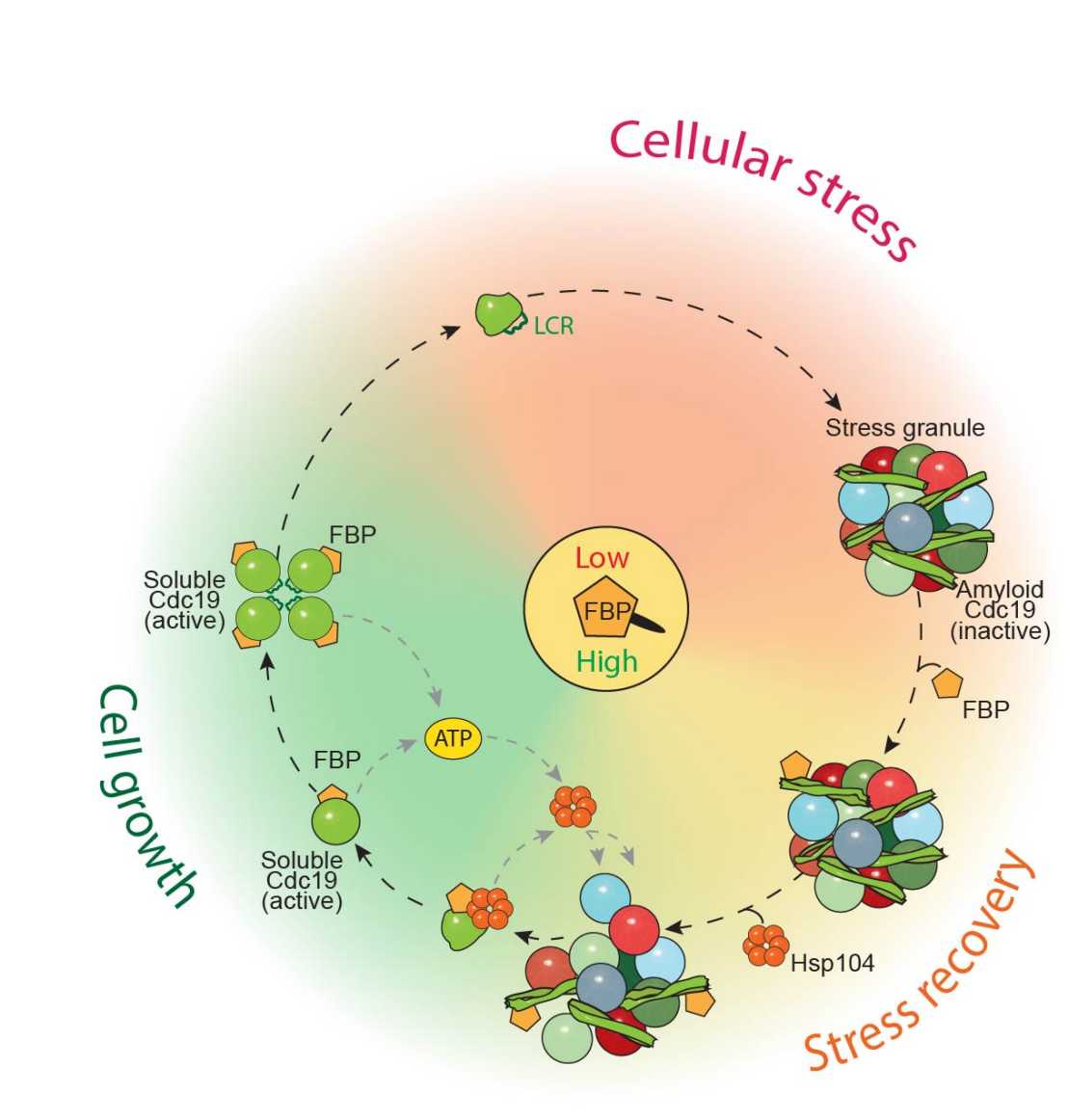
Cells respond to stress by blocking translation, rewiring metabolism, and forming transient mRNP assemblies called stress granules (SGs). After stress release, re-establishing homeostasis requires energy-consuming processes. However, the molecular mechanisms whereby cells restore energy production to disassemble SGs and reinitiate growth after stress remain poorly understood.
The paper shows that, upon stress, the ATP-producing enzyme Cdc19 forms inactive amyloids, and that their rapid re-solubilization is essential to restore energy production and disassemble SGs. Cdc19 re-solubilization is initiated by the glycolytic metabolite fructose-1,6-bisphosphate (FBP), which directly binds Cdc19 amyloids and facilitates conformational changes that allow Hsp104 and Ssa2 chaperone recruitment. FBP then promotes Cdc19 tetramerization, which boosts its activity to further enhance ATP production and SG disassembly.
Together, these results describe a molecular mechanism essential for stress recovery, which directly couples metabolism with SG dynamics via regulation of Cdc19 amyloids
Link to the paper in external page "Nature Cell Biology".
Comments
No comments yet