Structural basis of calmodulin modulation of the rod cyclic nucleotide-gated channel
A "PNAS" paper coordinated by Jacopo Marino with contributions from the Schertler, Korkhov, Leitner, Picotti & Kaupp groups (PSI, IMBB, IMSB and Uni Bonn) reports the structure of the rod cyclic nucleotide-gated channel in complex with calmodulin. The combination of cryo-EM & structural proteomics explains crucial steps in visual signal transduction.
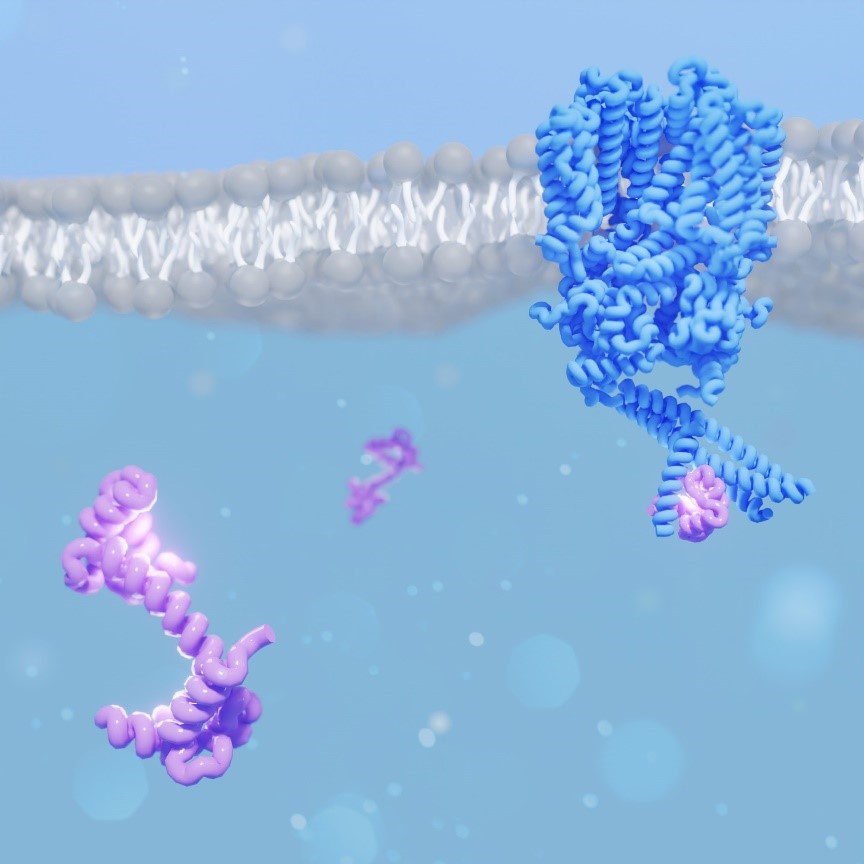
Calmodulin (CaM) regulates many ion channels to control calcium entry into cells, and mutations that alter this interaction are linked to fatal diseases. The structural basis of CaM regulation remains largely unexplored. In retinal photoreceptors, CaM binds to the CNGB subunit of cyclic nucleotide-gated (CNG) channels and, thereby, adjusts the channel’s cGMP sensitivity in response to changes in ambient light conditions. Here, Barret and Schuster et al. provide the first structural characterization for CaM regulation of a CNG channel by using a combination of single-particle cryo-electron microscopy and structural proteomics. CaM connects the CNGA and CNGB subunits resulting in structural changes both in the cytosolic and transmembrane regions of the channel. Crosslinking and limited proteolysis-coupled mass spectrometry mapped the conformational changes induced by CaM in vitro and in the native membrane. They propose that CaM is a constitutive subunit of the rod channel to ensure high sensitivity in dim light. Their mass-spectrometry-based approach is generally relevant for studying the effect of CaM on ion channels in tissues of medical interest, where only minute quantities are available.
Link to the paper in external page "PNAS".
Comments
No comments yet